Generic Product Launch
Varenicline Teva
Film-coated Tablets varenicline

Film-coated Tablets varenicline
Available on private prescription only.
Indications


Varenicline Teva 0.5 mg and Varenicline Teva 1 mg Film-coated Tablets (initiation pack) and Varenicline Teva 1 mg
Film-coated Tablets
Varenicline Teva is indicated for smoking cessation in adults.
Varenilcine 0.5mg and 1mg Film-Coated Tablets Abbreviated
Prescribing Information Presentation: Each film-coated tablet contains varenicline citrate equivalent to 0.5mg and 1mg varenicline. Indications: Varenicline is indicated for smoking cessation in adults. Dosage and administration: Oral use. Adults: The recommended dose is 1mg Varenicline twice daily following a 1-week titration (see SmPC for details). Children: Not recommended for use. Elderly: No dosage adjustment is necessary. Elderly patients are more likely to have decreased renal function, prescribers should consider the renal status of an elderly patient. Renal impairment: No dosage adjustment is necessary for patients with mild (estimated creatinine clearance >50ml/min and ≤80ml/min) to moderate (estimated creatinine clearance ≥30ml/min and ≤50ml/min) renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30ml/min), the recommended dose of Varenicline is 1mg once daily. Hepatic impairment: No dosage adjustment is necessary. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: Physiological changes resulting from smoking cessation, with or without treatment with Varenicline, may alter the pharmacokinetics or pharmacodynamics of some medicinal products, for which dosage adjustment may be necessary (examples include theophylline, warfarin and insulin). As smoking induces CYP1A2, smoking cessation may result in an increase of plasma levels of CYP1A2 substrates. Changes in behaviour or thinking, anxiety, psychosis, mood swings, aggressive behaviour, depression, suicidal ideation and behaviour and suicide attempts have been reported in patients attempting to quit smoking with Varenicline. Depressed mood, rarely including suicidal ideation and suicide attempt, may be a symptom of nicotine withdrawal. Clinicians should be aware of the possible emergence of serious neuropsychiatric symptoms in patients attempting to quit smoking with or without treatment. If serious
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie
neuropsychiatric symptoms occur whilst on Varenicline treatment, patients should discontinue Varenicline immediately and contact a healthcare professional for reevaluation of treatment. Smoking cessation, with or without pharmacotherapy, has been associated with exacerbation of underlying psychiatric illness (e.g. depression). In clinical trials and post-marketing experience there have been reports of seizures in patients with or without a history of seizures, treated with Varenicline. Varenicline should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold. At the end of treatment, discontinuation of Varenicline was associated with an increase in irritability, urge to smoke, depression, and/or insomnia in up to 3% of patients. In such instances, tapering should be considered. Patients taking Varenicline should seek immediate medical attention if they experience signs and symptoms of myocardial infarction or stroke. Interactions: Varenicline has no clinically meaningful drug interactions (see SmPC for further details). No dosage adjustment of Varenicline or co-administered medicinal products listed below is recommended. In vitro studies indicate that Varenicline is unlikely to alter the pharmacokinetics of compounds that are primarily metabolised by cytochrome P450 enzymes. Furthermore, since metabolism of Varenicline represents less than 10% of its clearance, active substances known to affect the cytochrome P450 system are unlikely to alter the pharmacokinetics of Varenicline, therefore a dose adjustment of Varenicline would not be required. Varenilcine is not known to affect the pharmacokinetics of metformin, digoxin, bupropion and warfarin. Co-administration of cimetidine, with Varenicline increased the systemic exposure of varenicline by due to a reduction in varenicline renal clearance. In patients with severe renal impairment, the concomitant use of cimetidine and Varenicline should be avoided. Pregnancy and lactation: As a precautionary
measure, it is preferable to avoid the use of varenicline during pregnancy. A decision on whether to continue/ discontinue breast-feeding or to continue/discontinue therapy with varenicline should be made taking into account the benefit of breast-feeding to the child and the benefit of varenicline therapy to the woman. Effects on ability to drive and use machines: Varenicline may have minor or moderate influence on the ability to drive and use machines. Varenicline may cause dizziness, somnolence and transient loss of consciousness, and therefore may influence the ability to drive and use machines. Adverse reactions: Diabetes mellitus, suicidal ideation, depression, hallucinations, psychosis, seizure, cerebrovascular accident, transient loss of consciousness, myocardial infarction, angina pectoris, tachycardia, atrial fibrillation, electrocardiogram ST segment depression, gastritis, haematemesis, severe cutaneous reactions including Stevens Johnson Syndrome and Erythema Multiforme, angioedema. Very Common: Nasopharyngitis, abnormal dreams, insomnia, headache, nausea. Common: Bronchitis, sinusitis, weight increased, decreased appetite, increased appetite, somnolence, dizziness, dysgeusia, dyspnoea, cough, gastrooesophageal reflux disease, vomiting, constipation, diarrhoea, abdominal distension, abdominal pain, toothache, dyspepsia, flatulence, dry mouth, rash, pruritus, arthralgia, myalgia, back pain, chest pain, fatigue, liver function test abnormal. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: In case of overdose, standard supportive measures should be instituted as required. Legal category: POM. Marketing Authorisation Number: 0.5mg PA1986/129/001, 1mg PA1986/129/002. Marketing
Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00084. Date of Preparation: July 2024.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Prescription Only Medicine. Further information is available on request or in the SmPC. Product Information also available on the HPRA website.
Date of Preparation: October 2024 | Job Code: Vcli-IE-00010
Call for papers: make your contribution to Hospital Professional News
Articles
Research Papers
Reviews
Programme Descriptions
Reports
Case Reports
Letters to Editor
Support fellow hospital professionals as well as aspiring junior professionals and early-year hospital pharmacists
Practice reports share innovations on any area of practice, including delivering clinical services, pharmacy administration, or new approaches to inform and engage with patients
Perspective articles focus on a specific field or discipline and discuss current advances or future directions, and may include original data as well as expert insight and opinions












Impact Award for Irish Researcher P5
Consultants show they are ‘Leading from the Front’ P8
New Chronic Disease Hubs launched in Ireland P10
Insights from the recent HIQA Inpatient Experience Survey P12
Hospital Pharmacists Education and Research Specialist Group P34
HSE published 2025 National Service Plan P37
Beaumont Hospital announce Strategic Plan P87
Respiratory Focus: Cystic Fibrosis P23
Respiratory Focus: Pulmonary Rehabilitation P25
CPD: Rheumatoid Arthritis P55
Oncology Focus: Breast Cancer Brain Metastases P65
P60
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only.
All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission.
IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd
Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
danielle@hospitalprofessionalnews.ie
ACCOUNTS
Fiona Bothwell
cs.ipn@btconnect.com
SALES EXECUTIVE
Jean Lonergan
jean@hospitalprofessionalnews.ie
+353 87 288 2371


In one of our lead news stories this month, we feature coverage from the recent Irish Hospital Consultant Association’s Symposium: “Implementing Sustainable Healthcare Solutions”, a landmark event that brings together leading international health experts to discuss practical, actionable measures to improve sustainability within the health service.
Reaffirming the IHCA’s commitment to fostering a greener, more sustainable future for Irish healthcare, the symposium featured a distinguished panel of international and Irish experts, sharing insights and practical sustainability strategies that have successfully been implemented in hospitals worldwide. It served as a platform for collaboration and knowledge-sharing, driving meaningful action to meet the twin challenges of sustainability and climate resilience.

Experts showcased evidence based solutions that can reduce the environmental footprint of healthcare practices while improving patient care and delivering savings for public finances, ensuring long-term benefits for both the planet and healthcare systems. Turn to page 8 for the full details.
SALES & TRAINING MANAGER
Amy Evans | amy@ipn.ie 0872799317

CONTRIBUTORS
Tracy O’Carroll | Dr Deirdre McDermott
Lorna Nellany | Nathan Scanlon
Nicola Delaney Foxe | Darren J. Walsh
Sarah Brown | Carina O’Brien
Ita Fitzgerald | Dr Orla Killeen
Dr Kathy Gately | Sharon O’Toole
Éanna J. Ryan | Ronan A. Cahill
Dr Sinead Toomey | Dr Damir Vareslija
Dr M Raheel Khan | Dr Louise Horrigan
Jemma Buchalter | Michaela J. Higgins
Roisin McAvera | Alessandra Allotta
Dr Daniela Ottaviani | Dr Jason McGrath
Olivia Iarmak | Tatiana Iarmak
Dr Janet Moyle | Dr Olwyn Conlon
Cara M Martin | John J O’Leary
Dermatology Registrar
Professor Mary Laing
Paddy McGeoghegan
Professor Leonie Young
Ms Katie E Johnston
Dr Samantha J Cushen
Yasmine Maati Chaibi
Professor Richard Conway
Professor Bryan Hennessy
Professor Jarushka Naidoo
Dr Diarmuid McLaughlin
Dr Salvatore Vaccarella


In other news, on page 12 Tracy O’Carroll, Deputy Director of the National Care Experience Programme at Health Information and Quality Authority gives readers an overview of the HIQA National Inpatient Experience Survey. Since its inception in 2017, the National Inpatient Experience Survey (NIES) has offered a vital glimpse into Ireland's acute healthcare services through the eyes of those who matter most, patients.
‘In 2024, 12,367 participants shared their perspectives on care across all 40 public acute hospitals. Encouragingly, 85% of patients rated their overall hospital experience as good or very good. Improvements were especially notable in discharge care, which historically has been the lowest scoring stage of care,’ she reflects.
This issue features not one, but two Special Focus sections covering Respiratory and Oncology Care. For the former, we have clinically contributed articles on Asthma Care, authored by Dr Deirdre McDermott, Respiratory Registrar, Galway University Hospital and Dr Sinead Walsh, Consultant Respiratory Physician, Galway University Hospital & Galway City Integrated Care Hub as well as articles covering Cystic Fibrosis, COPD, RSV and Idiopathic Pulmonary Fibrosis.
Our in-depth Oncology Focus includes Testicular Cancer, written by Dr M Raheel Khan, Consultant Medical Oncologist, St James’s Hospital, Dublin, Emerging Treatments for Colorectal Cancer by Dr Sinead Toomey and Professor Bryan Hennessy and Immunotherapy for Early-Stage Lung Cancer: A Game Changer authored by Professor Jarushka Naidoo Dr Janet Moyle.
I hope you enjoy the issue.

Mater Private Network is proud to announce a groundbreaking milestone in cardiac care, becoming the first institution globally to enrol a patient in the pioneering LUMINIZE clinical study. This first-in-human procedure, led by Professor Gábor Széplaki, Head of Cardiac Electrophysiology at Mater Private Network, was completed using the state-of-the-art VERAFEYE™ Navigation and Visualisation Platform from LUMA Vision Ltd. The VERAFEYE™ system revolutionises intracardiac imaging by combining advanced manoeuvrability with cutting-edge
imaging technology to deliver realtime, four-dimensional, CT-quality anatomical reconstructions in under 60 seconds.
With plans to enrol patients with complex heart conditions such as atrial fibrillation (AFib) and structural heart disease over the coming month, Mater Private Network is leading the charge for advanced cardiac care in Ireland.
Speaking about his role in the trial, Professor Széplaki, Head of Cardiac Electrophysiology at the Mater Private Network, remarked: "At Mater Private Network, we
are always striving to be at the forefront of medical innovation and our participation in this trial is a great example of how our cardiology centre, in collaboration with the researchers at the Cardiovascular Research Institute (CVRI) Dublin and RCSI University of Medicine and Health Sciences, has become a centre of excellence for research and clinical care here in Ireland.”
The inclusion of Mater Private Network patients in this trial highlights the hospital’s subspecialty expertise in this field, employing the most AFib
The European Association of Hospital Pharmacists Annual Congress takes place on 12-14th March 2025 in Copenhagen, Denmark with the theme of Person-Centred Pharmacy –Navigating Digital Health
Hospital pharmacists around Europe will again have the opportunity to share their expertise and latest developments in practice to advance patient outcomes through learning and nurturing partnerships and networks.
The EAHP Congress will bring hospital pharmacists all closer to change, progress and evaluation of what they deliver today to patients seeking pharmaceutical care across European hospitals and healthcare systems in complex digital infrastructures. Moreover, the congress takes place in
times of a generational reform of pharmaceutical legislation, marking the beginning of different approaches towards digital environment where patient-centred and seamless care interlinks, while securing safe healthcare delivery and optimising health outcomes.
What to expect:
• Live panel discussions by hospital pharmacy and healthcare experts
• Social events and Networking opportunities
• Poster Walk Sessions
• Exhibition area
• Access to session presentations after the Congress
• …and much more!
Mater Private Networking team have been celebrating a groundbreaking milestone in cardiac care, becoming the first institution globally to enrol a patient in the pioneering LUMINEZ
specialist cardiologists and treating the highest volume of AFib patients in Ireland. This trial, driven by Mater Private Network’s continued collaboration with the CVRI Dublin and RCSI, provides unparalleled access to innovative treatments previously inaccessible to Irish patients.
The VERAFEYE™ system offers 360-degree visualisation with enhanced depth and clarity, overcoming the limitations of traditional two-dimensional imaging, and enabling clinicians to navigate and visualise cardiac anatomy dynamically from all angles. Ultimately this technology provides crucial support for complex cardiac interventions enhancing accuracy, efficiency, and safety in advanced electrophysiology procedures.
Mater Private Network, as a leader in cardiac care here in Ireland, is proud to continue to support and engage with innovative advances in cardiac medicine, to improve patient outcomes both in Ireland and beyond.

Dr Jakub Gajewski, Research Programme Director at the RCSI Institute of Global Surgery, has been announced as the winner of the Impact Award in the Researcher of the Year (IRC legacy) Awards.
The awards were announced at an event on Wednesday, 15 January to celebrate the very best of Irish Research Council-funded researchers deemed to have made highly significant and valuable contributions to knowledge, society, culture and innovation.
Dr Gajewski was presented with the Impact Award for his leadership in advancing sustainable healthcare for underserved populations, particularly in sub-Saharan Africa. His leadership in two European Commission-funded projects, COST and SURG-Africa (20112021), implemented in Malawi, Zambia, and Tanzania, has been instrumental in addressing the global shortage of safe surgery. Additionally, through his involvement RCSI Institute of Global Surgery’s Akazi Project on breast cancer control in Malawi, he has promoted early detection, raised awareness, and improved access to screening in underserved communities.
Dr Jakub Gajewski named researcher of the year
Commenting on the award Dr Gajewski said: “Receiving the Impact Award is both an honour and a source of renewed energy. It is a powerful reminder that research is not solely about publishing papers but about driving meaningful, lasting change. I am deeply grateful to RCSI and to my incredible team and collaborators who have made this work possible.”
Peter Brown, Director, of Researcher Development in Research Ireland congratulated the Researcher of the Year (IRC legacy) awardees, adding: “The researchers being honoured today have demonstrated excellence in their many achievements within and beyond their disciplines. The awardees are wonderful examples of those who have gone above and beyond to bring new knowledge and understandings to the fore. At different stages in their research journey, they are enriching their respective fields and Ireland’s research and innovation system.”

In August 2024, the Irish Research Council (IRC) amalgamated with Science Foundation Ireland (SFI) to become Taighde Éireann –Research Ireland, the new national funding agency for research and innovation in Ireland. As the award recipients were previously funded by the Irish Research Council, this 2024 awards round have been made as Researcher of the Year (IRC legacy) Awards. Dr Gajewski
The Pharmaceutical Manufacturer’s Institute (PM) Annual Pharma Summit returns to Croke Park on Thursday 3rd April, 2025. This must-attend event will showcase a diverse range of industry and subject experts under the general theme of “evolving through change”.
This is the flagship event in the PMI’s calendar with over 300 attendees from across the industry along with a host of exhibitors. It provides an excellent platform to learn, connect and grow!
Speakers will include Professor Kingston Mills – Professor of Experimental Immunology and Director of Trinity Biomedical Sciences Institute at Trinity College Dublin, Fionnuala King – Chief Pharmacist at HSE, Dr Roisin Adams – Head of HTA Strategy and External Engagement at NCPE and Gary Keegan – CEO & Founder at Uppercut plus many more. Visit www.thepmi.com for further information.
is the Research Programme Director at RCSI’s Institute of Global Surgery. Building on extensive experience in surgical training, education and research partnerships in Africa, the RCSI Institute of Global Surgery works with local, national and international partners to improve access to high-quality, essential surgical care for underserved populations.

The Chief Medical Officer, Professor Mary Horgan, has launched ‘Pathways to WellbeingNational Mental Health Promotion Plan’. This is Ireland’s first National Mental Health Promotion Plan, fulfilling a policy commitment set out in both ‘Sharing the Vision: A Mental Health Policy for Everyone’ and in the ‘Healthy Ireland Strategic Action Plan’.
The Plan also responds to the need for population-level
mental health promotion, as demonstrated by the impact of COVID-19 pandemic restrictions. It seeks to strengthen individuals and the communities where they live while addressing the structural barriers to good mental health at societal level.
Professor Horgan said:
"The National Mental Health Promotion Plan is an important step towards achieving the vision
for Healthy Ireland where everyone can enjoy physical and mental health and wellbeing to their full potential, and where health is valued and supported at every level in society.
"Pathways to Wellbeing is a significant milestone on this journey. There is compelling international evidence that focusing on mental health promotion can have positive effects on wellbeing as well as reducing the risk for mental health
difficulties. The goals set out in the Plan focus on the key areas which can have a significant impact on promoting mental wellbeing at a population level."
This Plan was informed by international evidence and provides the foundation for the programme of work that will be required to deliver on the high-level goals and objectives. The next phase is the development of the cross-government implementation plan in 2025.
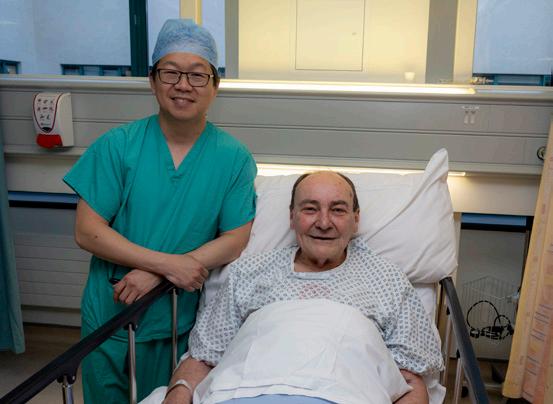
University Hospital Galway has become the first public hospital in the country to employ the 'Convergent Procedure', a groundbreaking approach for treating atrial fibrillation (Afib), putting it at the pinnacle of heart surgery.
Atrial fibrillation affects more than 1-2% of the population with increasing incidence in the over 65s, and if left untreated, can lead to blood clots, stroke, heart failure and other heart-relation complications.
Commenting Professor Alan Soo, Consultant Cardiothoracic Surgeon stated, “Afib is a condition where your heart beats irregularly (arrhythmia), usually much faster than normal and this is caused by disruptions in the electrical signals that regulate your heart, making it harder for the upper and lower chambers of the heart to work together.
“While medication or catheter ablation can successfully treat Afib, some patients’ symptoms
Professor Alan Soo, Consultant Cardiothoracic Surgeon at University Hospital Galway with patient Sean Rankin from Newcastle in Galway City. Sean was the first patient to undergo the 'Convergent Procedure' at University Hospital Galway
recur or worsen. Now with the convergent procedure, such patients’ have another treatment option allowing for minimal discomfort, lower risk of complications, faster recovery time and a shorter hospital stay.
“This minimally invasive procedure is a game changer for the treatment of atrial fibrillation and we are delighted to be the first public hospital in Ireland to offer this surgical procedure for patients with atrial fibrillation.”
The procedure is performed by a Cardiothoracic Surgeon and a Cardiac Electrophysiologist, where by radiofrequency energy is used to make ablations, or small lesions/burns on the heart that will effectively block the
Important new research is to be undertaken by a Consultant Neurologist at Tallaght University Hospital (TUH) to find out if blood tests can be used to diagnose Alzheimer’s disease in people with Down syndrome. Dr Antoinette O’Connor hopes this new study will also support future investigation of whether new medications to slow the progress of Alzheimer's could be effective for those with Down syndrome. This new study is necessary as people with Down syndrome have a significant risk of developing Alzheimer’s disease (AD). Recently new drugs have come on stream which have been shown to slow the progression of Alzheimer's for those in the early stages of the disease. The challenge for doctors is to determine if individuals with
Down syndrome can also benefit from these treatments.
In order to properly test these treatments, there will need to be carefully designed studies. Traditional tests, cerebrospinal fluid sampling, and brain scans are invasive and expensive, therefore it would be easier for participants if blood tests could be used to detect and track potential treatment effects.
Dr O’Connor says, “We are entering a new era in AD treatment– for the first time there are therapies that can slow disease progression. Frustratingly, people with Down syndrome have been routinely excluded from AD drug trials, despite urgent clinical need in this population. Therefore, we do not know if these potentially life-altering treatments work in Down syndrome.”
The TUH Consultant Neurologist says, “Robust clinical trials involving those with Down syndrome will need to be undertaken to track the changes in them caused by Alzheimer's disease. These measures of change are called biomarkers. Blood tests represent an ideal AD biomarker as they are cheap, accessible and repeatable.”
Several important questions must be answered before blood tests for AD can enter routine use for those with Down syndrome. Dr O’Connor's new research study will address some of these, specifically:
• What blood tests are the most promising for the detection of AD in Down syndrome?
• Do blood test levels vary from day to day and does this
irregular electrical signals or heartbeat and it is one of the most effective surgical techniques in treating individuals with persistent atrial fibrillation.
Galway resident, Sean Rankin was the first patient to undergo the Convergent Procedure, which was carried out by Professor Alan Soo, Consultant Cardiothoracic Surgeon and Dr Stephen Tuohy, Consultant Cardiac Electrophysiologist at University Hospital Galway.
Sean who had been suffering from atrial fibrillation for many years said, “I sought treatment for my condition following the success of my lung cancer surgery with Professor Soo. I’m doing very well and I am very pleased with the way everything went.
“I’d like to express my since appreciation to Professor Alan Soo, Dr Stephen Tuohy and my GP Dr Richie Baggott for everything they’ve done for me. This is a new beginning for me and I look forward to the future.”
Hospital Manager Chris Kane stated, "This is a first for University Hospital Galway, and we welcome this new treatment option for patients. It will significantly improve the patient experience, and we are delighted to have debuted this innovation here in Galway.”
variability impact their ability to diagnose AD and/or track change?
• What role does inflammation play in driving AD onset?
• How long, and how many people, are required to participate in AD clinical trials to show a treatment effect in Down syndrome?”
This new study will answer these questions by reviewing previous blood biomarker studies in people with Down syndrome and by collecting repeated blood samples from study participants. These blood samples will also enable Dr O’Connor and her team to investigate the role of inflammation in AD which could potentially open up new treatment avenues.


• Available in 800 IU tablets and 4000 IU tablets
• Daily flexible dosing of 800 IU - 4000 IU Vitamin D3

ABBREVIATED PRESCRIBING INFORMATION:
Desunin (colecalciferol), 800 IU & 4000 IU Tablets.
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Indications, Dosage and Administration:
Desunin 800 IU:
Desunin 800 IU is indicated for the prevention and treatment of vitamin D deficiency in adults and adolescents.
In addition to specific osteoporosis treatment of patients who are at risk of vitamin D deficiency, preferably in combination with calcium
Desunin 4000 IU:
Desunin 4000 IU is indicated for the treatment of vitamin D deficiency in adults and adolescents.
Vitamin D deficiency is defined as serum levels of 25-hydroxycolecalciferol (25(OH)D) < 25 nmol/l.
Recommended dose: One tablet per day.
The dose should be adjusted dependent upon desirable serum levels of 25-hydroxycolecalciferol (25(OH)D), the severity of the disease and the patient´s response to treatment.
The daily dose should not exceed 4000 IU.
Pediatric population
The safety and efficacy of Desunin in children under 12 years have not been established.
Dosage in hepatic impairment
No dose adjustment is required.
Dosage in renal impairment
Desunin should be used with caution in patients with renal impairment (see section 4.4 of the SmPC).
Administration: The tablets can be swallowed whole or crushed. The tablets can be taken with food.
Presentation: Tablets
Contraindications:
• Diseases and/or conditions resulting in hypercalcaemia or hypercalciuria.
• Nephrolithiasis
• Nephrocalcinosis
• Hypervitaminosis
D
• Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SmPC
Warnings and precautions:
Desunin should be prescribed with caution to patients suffering from sarcoidosis
metabolised normally and other forms of vitamin D may therefore be needed. The content of vitamin D (800 IU or 4000IU) in Desunin should be considered when prescribing other
excretion frequently.
Excipients: Desunin contain sucrose, isomalt and sodium. Patients with rare hereditary problems of fructose intolerance, glucose-galactose
This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.

insufficiency should not take this medicine.
Interactions with other medicinal products and other forms of interactions: Thiazide diuretics reduce the urinary excretion of calcium. Due to the increased risk of hypercalcaemia, serum calcium should be regularly monitored during concomitant use of thiazide diuretics.
Concomitant use of phenytoin or barbiturates may reduce the effect of vitamin D since the metabolism increases.
Excessive dosing of vitamin D can induce hypercalcaemia, which may increase the risk of digitalis toxicity and serious arrhythmias due to the additive inotropic effects. The electrocardiogram (ECG) and serum calcium levels of patients should be closely monitored.
Glucocorticoid steroids may increase vitamin D metabolism and elimination. During concomitant use, it may be necessary to increase the dose of Desunin tablets.
Simultaneous treatment with orlistat or ion exchange resins such as cholestyramine or laxatives such as paraffin oil may reduce the gastrointestinal absorption of vitamin D.
Fertility, pregnancy and lactation:
Fertility - There are no data on the effect of Desunin on fertility. However, normal endogenous levels of vitamin D are not expected to have any adverse effects on fertility.
Pregnancy - Desunin should be used during pregnancy, only in the case of a vitamin D deficiency. Desunin is not recommended during pregnancy in patients without a vitamin D deficiency as the daily intake should not exceed 600 IU vitamin D.
Studies in animals have shown reproductive toxicity of high doses of vitamin D (see section 5.3 of the SmPC).
There are no indications that vitamin D at therapeutic doses is teratogenic in humans.
Breast-feeding - Vitamin-D can be used during breast-feeding. Vitamin D3 passes into breast milk. This should be considered when giving additional vitamin D to the child.
Undesirable effects:
Very common (≥1/10): None
Common (>1/100, <1/10): None
For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC.
Reporting of adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie.
Adverse reactions/events should also be reported to the marketing authorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267.
Legal Category: Product subject to prescription which may be renewed (B)
Marketing Authorisation Number: PA23355/009/001, PA23355/009/004
Marketing Authorisation Holder: Viatris Healthcare Limited, Damastown Industrial Park, Mulhuddart Dublin 15, DUBLIN Ireland
Full Prescribing Information available on request from: Viatris, Dublin 17. Email: enquiry.ire@viatris.com.
Date of revision of Abbreviated Prescribing Information: 30 June 2023
Reference Number: IE-AbPI-Desunin-v005

Hospital Consultants and healthcare experts gathered recently for the Irish Hospital Consultant Association’s Symposium: “Implementing Sustainable Healthcare Solutions”, a landmark event that brings together leading international health experts to discuss practical, actionable measures to improve sustainability within the health service.
Reaffirming the IHCA’s commitment to fostering a greener, more sustainable future for Irish healthcare, the symposium featured a distinguished panel of international and Irish experts, sharing insights and practical sustainability strategies that have successfully been implemented in hospitals worldwide. It served
as a platform for collaboration and knowledge-sharing, driving meaningful action to meet the twin challenges of sustainability and climate resilience.
Speakers included Professor Hugh Montgomery, Professor of Intensive Care Medicine at the University College of London and Co-Chair of the Lancet Countdown, Dr Brian O’Connell, Emergency Medicine Consultant and Sustainable Healthcare / Climate Risk Lead, NSW, Australia, Dr Cathy Burke, Consultant Obstetrician/ Gynaecologist and Chairperson of the Green Group, Cork University Maternity Hospital, and Dr Philip Crowley, National Director for Wellbeing, Equality, Climate and Global Health in the HSE.
Experts showcased evidencebased solutions that can reduce the environmental footprint of healthcare practices while improving patient care and delivering savings for public finances, ensuring long-term benefits for both the planet and healthcare systems. As much as one third of health spending is not considered “high value” or effective care, such as unnecessary lab testing or inappropriate prescribing. Simple, practical changes can make a difference. Attendees at the IHCA symposium heard a number of practical, tried and tested measures, which have been implemented to great success
IHCA President, Prof. Gabrielle Colleran opened the 'Implementing Sustainable Healthcare Solutions' symposium, welcoming attendees and speaking about the need for a focus on sustainability in healthcare
in Ireland and in other countries, such as the successful “gloves off” campaign employed to great effect in the UK and Australia.
Addressing the Health Impacts of Climate Change
Discussions also explored how climate change is impacting public health, emphasising the role of healthcare professionals in mitigating these effects through proactive, sustainable practices. Climate change has been found to be inextricably linked to poor public health outcomes, with hospitalisations attributable to air pollution accounting for 63,572 bed days in Ireland between 20162019. Likewise, Ireland could see an 18-fold increase in the number of heat-related deaths, and almost a doubling in cold-related deaths in our ageing population, by 2100 due to climate change.
IHCA President Gabrielle Colleran highlighted the importance of this initiative saying, “Hospital consultants are uniquely positioned to lead the charge in integrating sustainable practices across our healthcare system. This symposium is a positive, ground-up movement, driven by the expertise and innovation of consultants who are committed to creating a healthier, more sustainable future for patients, the healthcare sector, and society as a whole.”
The IHCA invites healthcare professionals, policymakers, and stakeholders to join this critical conversation. Together, we can ensure Irish healthcare becomes a leader in tackling sustainability challenges while maintaining the highest standards of patient care.

Dr
Philip Crowley, National Director, HSE Wellbeing, Equality, Climate and Global Health. Dr Crowley discussed the HSE's Climate Action Strategy and the importance of a cohesive approach to sustainability in the health service

Jared Gormley, Head of the HSE Spark Innovation Programme also spoke at the symposium. The Spark Innovation Programme is a frontline, staff-led initiative that seeks to support, promote and recognise innovation amongst healthcare staff
“This symposium is a positive, ground-up movement, driven by the expertise and innovation of consultants who are committed to creating a healthier, more sustainable future for patients, the healthcare sector, and society as a whole.”

The Irish Hospital Consultants Association (IHCA) is calling for the forthcoming Programme for Government to include the implementation of the recommendations of the Department of Health expert group regarding reform of clinical negligence claims.
A Department of Health expert group, chaired by Professor Rhona Mahony, recently recommended various new approaches and mechanisms, including the introduction of pre-action protocols, with sanctions for any party who fails to adhere to them.
As outlined in the report, the primary driver of the rising cost of claims is the cost of care in a relatively small number of very serious injury claims. Over 50% of healthcare litigation costs come from just 2% of claims, mainly involving severe cases like perinatal brain injury and cerebral palsy.
Pre-action protocols have resulted in reduced legal costs in other jurisdictions, and it is anticipated that their introduction in Ireland would have a similar effect and lead to swifter resolution of claims.
Implementing these reforms will be crucial to accelerating the resolution times of claims, reducing the cost of litigation, and sparing patients and families from prolonged and stressful legal processes.
Consultants have welcomed the government’s commitment, on the report’s launch, to establish a working group to ensure that its recommendations were “implemented without delay”. The IHCA looks forward to supporting the next Minister for Health in delivering on this commitment.
Professor Gabrielle Colleran said, “One of the first priorities of the next government should be the swift introduction of pre-action protocols to curtail the surging costs of litigation and to provide speedier resolution of claims for patients.
“The present litigation system in Ireland is excessively adversarial, with clinical negligence claims often taking several years to resolve. This is just adding to the distress faced by patients, their families and healthcare professionals.
“The current protracted claims processes are not fit for purpose and are resulting in increased legal costs that are among the highest in the world.
“Importantly, such costs represent a significant drain on public funds and draw resources away from the provision of healthcare. By working together to implement these reforms, we can not only control rising costs but also ensure better outcomes for patients, healthcare professionals, and the public. The IHCA is eager to engage with the next government to make this vision a reality."

People living with diabetes in the West, who are benefiting from faster access to specialised care as a result of the new HSE Chronic Disease Hubs, have praised the enhancements in diabetes care. The move away from hospital-based care means that patients have access to highly specialised clinical teams, closer to their homes.
There are HSE Integrated Care Hubs for individuals with chronic diseases across Counties Galway, Mayo and Roscommon. People with chronic diseases like Asthma, COPD, Type 2 Diabetes or Cardiovascular Disease can be referred directly to a local hub by their GP, instead of being referred to a hospital-based service.
The service is delivered across three hubs: the West Galway and City Integrated Care Hub provides clinics in Newcastle, Moycullen, Carraroe, Clifden, Oughterard, Doughiska and Renmore, the East Galway Roscommon Integrated Care Hub, located in Ballinasloe, provides clinics in Athenry, Ballinasloe, Loughrea, Tuam, Castlerea and Roscommon town and the Mayo Integrated Care Hub, located in Castlebar provides clinics in Achill, Ballinrobe, Belmullet, Castlebar, Claremorris, Swinford and Westport.
This is a major shift in the way healthcare services are delivered and a core component of Sláintecare; Ireland’s strategy for reforming the health and social care system
At the Moycullen Integrated Care Hub, from left, Dr Tomás Griffin, Lead Consultant Diabetologist for the West Galway and City Integrated Care Hub and Martin McDonagh, person living with diabetes
Roscommon Integrated Care Hub led by Dr Tomás Griffin and Dr Abdullah Abdullah.
Over the last year significant progress has been achieved in reducing hospital waiting lists through the work of the hubs, hospital teams and other initiatives, demonstrating the impact and benefits of an integrated model of care for patients. Between July 2023 and October 2024, Galway University Hospitals had an 83% reduction in waiting lists, while Roscommon University Hospital saw a 71% reduction.
Being able to treat patients in community-based specialist centres leads to an overall reduction in hospital waiting lists and the diabetes service in particular has yielded very positive results across the region.
Between January and September this year over 10,000 appointments were carried out across the three hubs, where people living with diabetes were seen and treated by multidisciplinary teams including diabetic nurses, podiatrists and dieticians.
An additional 2,827 consultantled appointments in diabetes care were carried out in the West Galway and City Integrated Care Hub and East Galway
The HSE in partnership with people living with HIV, has launched ‘You, Me and HIV,’ a new nationwide campaign to address misconceptions around HIV and help reduce the stigma experienced by people living with HIV in Ireland. The campaign features people living with HIV and their loved ones. The campaign was developed by the HSE in close collaboration with people living with HIV and community and voluntary groups.
Research** has found that late diagnosis is often related to fear of diagnosis and stigma, highlighting the need to reduce stigma around HIV and testing. The latest figures*** from the Health Protection Surveillance Centre (HPSC) show a decrease in
the rate of first-time HIV diagnoses in Ireland in 2023. However, nearly two in five people (39%) were diagnosed late.
Knowing your HIV status allows you to get access to essential treatment and care to live a healthy life. Advancements in treatment for HIV, mean that people on effective treatment cannot pass HIV to sexual partners. In a recent survey, 71% of Irish adults were unaware of these advances in treatment. In addition, effective treatment in pregnancy prevents HIV transmission to babies.
HIV activists, and ‘Poz Vibes’ podcast creators Enda McGrattan, also known as drag star Veda Lady, and Robbie Lawlor, are among the people involved in the campaign.
HIV Fact Check:
• HIV, regardless of whether or not a person is on treatment, is not passed on from kissing, or from using the same cups, plates, forks or toilet seats. You can’t get HIV from shaking someone’s hand or giving them a hug.
• People on effective treatment cannot pass HIV to sexual partners. When a person living with HIV is on effective treatment, the viral load is so low that it is not detected in their blood. This is often referred to as 'undetectable' equals 'untransmittable' (U=U).
• People living with HIV on effective treatment can have healthy pregnancies and go on to deliver healthy babies without
Lead Consultant Diabetologist for the West Galway and City Integrated Care Hub, Tomás Griffin said, “This service places the person living with diabetes at the heart of care, empowering each person to manage their condition with confidence through timely, accessible support close to home.
“By reducing hospital wait times and offering direct access to a multidisciplinary team that includes, diabetes specialist nurses, an advanced nurse practitioner, podiatrists, a physiotherapy led exercise programme, and a dietitianwe work with individuals and their GPs to develop personalised care plans that foster improved health outcomes and greater self-management, all within a convenient, communitybased setting.”
passing on the virus. Effective treatment in pregnancy prevents infection in babies. HIV is not a reason to avoid pregnancy.
• HIV and AIDS are not the same thing. HIV is a virus that attacks the human immune system weakens its ability to fight infection and disease. AIDS describes the group of illnesses that you can get in the late stage of HIV infection. Most people with HIV will not develop AIDSrelated illnesses because of the advancements in treatments.
• With effective treatment, those living with HIV can go on to live a long, and healthy life.
For more resources on HIV see hse.ie/HIV

When statins* and ezetimibe are not enough, add on once daily oral bempedoic acid earlier, to help your patients go even further.1,2Δ ® ®
* Concomitant use with simvastatin >40 mg daily is contraindicated; please refer to the relevant SmPC for more information.1,2

Δ NILEMDO® and NUSTENDI® are indicated in adults with established, or at high risk for, ASCVD to reduce CV risk by lowering LDL-C levels, as an adjunct to correction of other risk factors, who are on maximally-tolerated statins, or statin-intolerant, or statin-contraindicated with or without ezetimibe or not adequately controlled with ezetimibe treatment.1,2
NILEMDO (bempedoic acid) 180 mg / NUSTENDI (bempedoic acid/ezetimibe) 180 mg/10 mg filmcoated tablets
Abbreviated Prescribing Information Refer to Summary of Product Characteristics (SmPC) prior to prescribing.
Presentation: Each Nilemdo film-coated tablet contains 180 mg bempedoic acid. Each Nustendi film-coated tablet contains 180 mg of bempedoic acid and 10 mg of ezetimibe. Indications: Hypercholesterolaemia and mixed dyslipidaemia: Nilemdo/Nustendi are indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia as an adjunct to diet: In combination with a statin (Nilemdo: or statin with other lipid-lowering therapies) in patients unable to reach low-density lipoprotein cholesterol (LDL-C) goals with the maximum tolerated dose of a statin; alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant; or for whom a statin is contraindicated (Nustendi: and are unable to reach LDL-C goals with ezetimibe alone). Cardiovascular disease: In adults with established or at high risk for atherosclerotic cardiovascular disease to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors: in patients on a maximum tolerated dose of a statin with or without ezetimibe or; alone in patients who are either statin-intolerant, or for whom a statin is contraindicated (Nustendi: or in patients already being treated with the combination of bempedoic acid and ezetimibe as separate tablets with or without statin.) Posology and method of administration: The recommended dose is one tablet of 180 mg Nilemdo or 180 mg/10 mg Nustendi taken once daily, with or without food. Tablet should be swallowed whole. Concomitant simvastatin therapy: When Nilemdo/Nustendi are co-administered with simvastatin, simvastatin dose should be limited to 20 mg daily (or 40 mg daily for patients with severe hypercholesterolaemia and high risk for cardiovascular complications, who have not achieved their treatment goals on lower doses and when the benefits are expected to outweigh the potential risks). Coadministration with bile acid sequestrants: Dosing of Nustendi should occur either at least 2 hours before or at least 4 hours after administration of a bile acid sequestrant.
Paediatric Population: The safety and efficacy of Nilemdo/Nustendi in children aged less than 18 years have not yet been established. Contraindications: Hypersensitivity to the active substance or any of the excipients (see SmPC); pregnancy; breast-feeding; concomitant use with simvastatin > 40 mg daily. When Nustendi is co-administered with statin in patients with active liver disease or unexplained persistent elevations in serum transaminases; when Nustendi is co-administered with a statin, consult the SmPC for that particular statin therapy. Warnings and precautions: Potential risk of myopathy with concomitant statins: Bempedoic acid increases plasma concentrations of statins. Patients receiving Nilemdo and a statin should be monitored for adverse reactions that are associated with high doses of statins. Statins occasionally cause myopathy. In rare cases, myopathy may take the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria and can lead to fatality. In post marketing experience with ezetimibe, very rare cases of myopathy and rhabdomyolysis were reported. Most patients who developed rhabdomyolysis were taking a statin with ezetimibe. Patients receiving Nilemdo/Nustendi and a statin should be advised of the potential increased risk of myopathy and told to report promptly any unexplained muscle pain, tenderness, or weakness. If such symptoms occur, a lower maximum dose of the same statin or an alternative statin, or discontinuation of Nilemdo/Nustendi and initiation of an alternative lipid-lowering therapy should be considered under close monitoring of lipid levels and adverse reactions. If myopathy is confirmed by creatine phosphokinase (CPK) > 10× upper limit of normal (ULN), immediately discontinue Nilemdo/ Nustendi and any statin. Increased serum uric acid: Bempedoic acid may raise serum uric acid due to inhibition of renal tubular OAT2 and may cause or exacerbate hyperuricaemia and precipitate gout in patients with history of gout or predisposed to gout. Discontinue Nilemdo/Nustendi if hyperuricaemia accompanied with symptoms of gout appear. Elevated liver enzymes: Liver function tests should be performed at initiation of therapy. Discontinue Nilemdo/Nustendi if increase in transaminases > 3× ULN
persists. Renal impairment: Additional monitoring for adverse reactions may be warranted in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) or patients with ESRD on dialysis. Hepatic impairment: Periodic liver function tests should be considered for patients with severe hepatic impairment (Child-Pugh C) taking Nilemdo. Nustendi is not recommended in moderate to severe hepatic impairment (Child-Pugh B and C) due to unknown effects of increased exposure to ezetimibe. Fibrates: If cholelithiasis is suspected in a patient receiving Nustendi and fenofibrate, gallbladder investigations are indicated, and therapy should be discontinued. Ciclosporin: Caution when initiating Nustendi in the setting of ciclosporin. Ciclosporin concentrations should be monitored. Anticoagulants: Appropriately monitor INR if Nustendi is added to warfarin, other coumarin anticoagulants, or fluindione. Contraception measures in women of child-bearing potential: Before initiating treatment in women of child-bearing potential appropriate advice on effective methods of contraception should be provided, and effective contraception initiated. Patients taking oestrogen-based oral contraceptives should be advised to stop Nilemdo/Nustendi before stopping contraceptive measures if planning to become pregnant. Excipients: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take Nilemdo/Nustendi as it contains lactose. Patients at high risk of cardiovascular disease: Evidence for the use of the fixed combination medicinal product of bempedoic acid with ezetimibe in patients at high risk of cardiovascular disease is only available for the lipid-lowering effect in absence of any cardiovascular risk reduction estimation for ezetimibe in primary prevention patients. Driving and use of machines: Nustendi has minor influence on ability to drive and use machines. Dizziness has been reported. Interaction with other medicinal products: Refer to SmPC for full information on interactions. Adverse reactions: Nilemdo: Common (≥ 1/100 to < 1/10): Glomerular filtration rate decreased, anaemia, gout, hyperuricaemia (includes blood uric acid increased), AST increased, pain in extremity. Uncommon (≥ 1/1,000 to < 1/100): weight decreased, haemoglobin decreased, ALT increased, liver function test increased, blood creatinine increased, blood urea increased, Consult Nilemdo SmPC in relation to other adverse reactions. Nustendi: Common (≥ 1/100 to < 1/10): Glomerular filtration rate decreased, anaemia, decreased haemoglobin, hyperuricaemia (includes uric acid increased), decreased appetite, dizziness, headache, hypertension, cough, constipation diarrhoea, abdominal pain, nausea, dry mouth, flatulence, gastritis, liver function test increased (includes liver function test abnormal), back pain, muscle spasms, myalgia, pain in extremity, arthralgia, blood creatinine increased, fatigue, asthenia, gout, AST increased (for bempedoic acid), blood CPK increased. Uncommon (≥ 1/1,000 to < 1/100): weight decreased, ALT increased, blood urea increased, hot flush, dyspepsia, gastrooesophageal reflux disease, AST increased (for ezetimibe), GGT increased, pruritus (with statin), neck pain, muscular weakness (with statin), chest pain, pain, oedema peripheral (with statin). Frequency not known: Thrombocytopaenia, hypersensitivity (including rash, urticaria, anaphylaxis, angio-oedema), depression, paraesthesia (with statin), dyspnoea, pancreatitis, hepatitis, cholelithiasis, cholecystitis, erythema multiform, myopathy / rhabdomyolysis. Consult Nustendi SmPC in relation to other adverse reactions. Legal Classification: POM. Package quantity, marketing authorisation (MA) number: Nilemdo 28 tablets: EU/1/20/1425/002. Nustendi 28 tablets: EU/1/20/1424/002. MA Holder: Daiichi Sankyo Europe GmbH, Zielstattstrasse 48, 81379 Munich, Germany. Further information available on request from Daiichi Sankyo Ireland Ltd. D09 YF97. Telephone: (01) 489 3000. Fax: (01) 489 3033. Email: medinfo@daiichi-sankyo.ie Date of Preparation: December 2024. JOB ID: IE/BEM/12/24/0005.
Healthcare professionals are asked to report any suspected adverse reactions via the HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse events or a product complaint about a Daiichi Sankyo medicine can also be directly reported to Daiichi Sankyo Ireland Ltd. D09 YF97 by telephone: +353 (1) 4893000.
References: 1. NILEMDO®. Summary of Product Characteristics. Available from: https://www.medicines.ie/medicines/nilemdo-180mg-film-coated-tablets-36336/spc [Accessed December 2024]. 2. NUSTENDI®. Summary of Product Characteristics. Available from: https://www.medicines.ie/medicines/nustendi-180mg-10mg-film-coated-tablets-36337/spc [Accessed Date: December 2024] IE/BEM/12/24/0002 | Date of preparation: January 2025

The National Inpatient Experience Survey is part of the work of the National Care Experience Programme, which is a joint initiative from the Health Information and Quality Authority (HIQA), the Health Service Executive (HSE) and the Department of Health.
Since its inception in 2017, the National Inpatient Experience Survey (NIES) has offered a vital glimpse into Ireland's acute healthcare services through the eyes of those who matter most, patients.
The National Inpatient Experience Survey is part of the work of the National Care Experience Programme, which is a joint initiative from the Health Information and Quality Authority (HIQA), the Health Service Executive (HSE) and the Department of Health. The initiative has continuously sought to improve the quality of health and social care services by
asking people in Ireland about their care experiences and acting on their feedback.
This year's findings underscore the continued importance of patient-centred care, revealing both commendable strides and persistent challenges.
Patient experience encompasses everything from being treated with dignity and respect to understanding discharge instructions. Studies consistently show that positive patient experiences correlate with better health outcomes and enhanced staff satisfaction. Listening to
Written by Tracy O’Carroll, Deputy Director of the National Care Experience Programme at Health Information and Quality Authority
patients is not merely a courtesy; it is essential to building trust and driving quality improvement in healthcare.
The National Inpatient Experience Survey serves as a compass, helping policymakers and healthcare professionals identify areas that require attention and improvement. Insights gathered over time allow us to measure the impact of initiatives, fostering transparency and accountability within the healthcare system.
Highlights from the 2024 Survey In 2024, 12,367 participants shared their perspectives on care across all 40 public acute hospitals. Encouragingly, 85% of patients rated their overall hospital experience as good or very good. Improvements were especially notable in discharge care, which historically has been the lowestscoring stage of care. The national score for discharge rose, with gains observed in areas like receiving information on managing conditions post-discharge and clarity around medication.
This progress reflects targeted efforts by the Health Service Executive (HSE) since 2017, to enhance the provision of clearer discharge instructions, improve communication about medications, and improve aftercare planning. Yet, significant gaps remain. For instance, 15% of patients reported inadequate explanations about their medicines or their condition after leaving the hospital.
The majority of patients reported feeling treated with respect and dignity and expressed trust in hospital staff. New questions added to this year's survey
revealed high levels of confidence in the safety of care received.
However, certain areas still demand urgent attention. Some patients with worries or fears reported they could not find a member of hospital staff to talk to during their hospital stay. Furthermore, younger patients, women, and individuals with disabilities consistently reported less positive experiences compared to other groups, highlighting inequities that must be addressed.
Discharge care exemplifies the balance between progress and challenges. While communication between staff and families during discharge has improved, many patients still struggle with inadequate preparation for recovery. One respondent remarked, “I live alone, and nobody asked how I’d manage post-discharge.” This sentiment underlines the need for a more holistic approach, ensuring all patients feel supported during this vulnerable transition.
Sustaining Momentum
Moving forward, the NIES will be conducted every other year, providing an ongoing opportunity to identify what is working well and what requires further improvements.
But real change requires sustained commitment. The implementation of quality improvement plans informed by patient feedback is critical, with adequate resources, monitoring mechanisms and stakeholder collaboration required to ensure success.
Conclusion: A Call to Action
The 2024 National Inpatient Experience Survey offers a patient-centred perspective of the healthcare system. It provides not only a snapshot of what is working well but also creates a compelling mandate for change. It reminds us that patients' voices are central to healthcare reform.
By prioritising patient voices, we can create a healthcare system that embodies safety, dignity, and inclusion. These findings are a call to action for all stakeholders to use these insights to shape a healthcare system worthy of the trust placed in it by every patient and ensure that every patient feels safe, respected, and cared for at every stage of their care journey.
2,3

LIXIANA® was developed with the ageing NVAF patient in mind.1,4 By offering a unique combination of clinical1,4,5 and practical2,6 benefits, LIXIANA® may help reduce the complexity in managing stroke prevention in your ageing NVAF patients.2
LIXIANA® is a once-daily DOAC indicated for:
• Prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) with one or more risk factors, such as congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischaemic attack (TIA).2
• Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.2
References: 1. Giugliano et al. N Engl J Med. 2013;369:2093-2104. 2. LIXIANA® Summary of Product Characteristics. Available at: https://www.medicines.ie. Accessed June 2024. 3. Kirchhof P et al. Int J Cardiol. 2023. 4. Kato ET et al. J Am Heart Assoc 2016;5(5). pii: e003432. 5. Ruff CT et al. Lancet 2015;385(9984):2288-95. 6. Steffel J et al. Eur Heart J 2018;39:1330-1393.
LIXIANA® (edoxaban) 60 mg / 30 mg / 15 mg film-coated tablets prescribing information
See Lixiana Summary of Product Characteristics (SmPC) prior to prescribing for full list of adverse events
Presentation: 60 mg (yellow) / 30 mg (pink) / 15mg (orange) edoxaban (as tosilate) film-coated tablets.
Indications: Prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack (TIA). Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. Posology and method of administration: NVAF: Recommended dose is 60 mg edoxaban once daily with or without food. Continue therapy long term. VTE: Recommended dose is 60 mg edoxaban once daily with or without food following initial use of parenteral anticoagulant for at least 5 days. These two treatments should not be administered simultaneously, as per SmPC. Short duration of therapy (at least 3 months) should be based on risk profile of the patient. For NVAF and VTE the recommended dose is 30 mg edoxaban once daily in patients with one or more of the following: moderate or severe renal impairment (creatinine clearance (CrCL) 15-50 mL/min); low body weight ≤ 60 kg; concomitant use of the P-glycoprotein (P-gp) inhibitors, ciclosporin, dronedarone, erythromycin, or ketoconazole. The 15 mg dose of edoxaban is not indicated as monotherapy and should only be used during a switch from edoxaban to VKA in certain patients (see SmPC for full details). Edoxaban can be initiated or continued in patients who may require cardioversion. For transoesophageal echocardiogram guided cardioversion in patients not previously treated with anticoagulants, edoxaban should be started at least 2 hours before cardioversion to ensure adequate anticoagulation. Cardioversion should be performed no later than 12 hours after the dose of edoxaban on the day of the procedure. Confirm prior to cardioversion that the patient has taken edoxaban as prescribed. Paediatric population: Edoxaban is not recommended for use in children and adolescents from birth to 18 years of age with confirmed VTE (PE and/or DVT) event as the efficacy has not been established. If a dose of edoxaban is missed, the dose should be taken immediately and then continued once daily on the following day. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Clinically significant active bleeding. Hepatic disease associated with coagulopathy and clinically relevant bleeding risk. Lesion or condition, if considered to be a significant risk for major bleeding including current or recent gastrointestinal (GI) ulceration, presence of malignant neoplasms at high risk of bleeding, recent brain or spinal injury, recent brain, spinal or ophthalmic surgery, recent intracranial haemorrhage, known or suspected oesophageal varices, arteriovenous malformations, vascular aneurysms or major intraspinal or intracerebral vascular abnormalities. Uncontrolled severe hypertension. Concomitant treatment with any other anticoagulants e.g. UFH, low molecular weight heparins, heparin derivatives (fondaparinux, etc.), VKA or DOACs except under specific circumstances of switching oral anticoagulant therapy or when UFH is given at doses necessary to maintain an open central venous or arterial catheter. Pregnancy and breast-feeding. Special warnings and precautions for use: Haemorrhagic risk: Caution in patients with increased risk of bleeding such as elderly on ASA. Discontinue if severe haemorrhage occurs. The anticoagulant effect of edoxaban cannot be reliably monitored with standard laboratory testing. A specific anticoagulant reversal agent for edoxaban is not available. Haemodialysis does not significantly clear edoxaban. Renal impairment: CrCl should be monitored at the initiation of edoxaban and afterwards when clinically indicated. Not recommended in patients with end stage renal disease or on dialysis. Renal function and NVAF: A trend towards decreasing efficacy with increasing CrCl was observed for edoxaban
IE/EDX/02/24/0006
Date of preparation: August 2024
compared to well-managed warfarin. Edoxaban should only be used in patients with NVAF and high CrCl after a careful benefit risk evaluation. Hepatic impairment: Not recommended in severe hepatic impairment. Caution in mild or moderate hepatic impairment. Caution in patients with elevated liver enzymes (ALT/ AST > 2 x ULN) or total bilirubin ≥ 1.5 x ULN. Perform liver function testing prior to initiation and then periodically monitor for treatment beyond 1 year. Surgery or other interventions: discontinue edoxaban as soon as possible and preferably at least 24 hours before the procedure. If procedure cannot be delayed, the increased risk of bleeding should be weighed against urgency of the procedure. Restart edoxaban as soon as haemostasis achieved. Prosthetic heart valves and moderate to severe mitral stenosis: Not recommended. Haemodynamically unstable PE patients or patients who require thrombolysis or pulmonary embolectomy: Not recommended. Patients with active cancer: Not recommended in treatment and/or prevention of VTE. Patients with a history of thrombosis diagnosed with antiphospholipid syndrome: DOACs including Edoxaban are not recommended. Drug interactions: Concomitant use of the P-gp inhibitors ciclosporin, dronedarone, erythromycin, or ketoconazole requires edoxaban dose reduction to 30mg. Edoxaban should be used with caution with concomitant P-gp inducers (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s Wort). Concomitant high dose ASA (325 mg) or chronic NSAIDs is not recommended. Concomitant ASA at doses > 100 mg and < 325 mg should be under medical supervision only. Very limited experience with dual antiplatelet therapy or fibrinolytics. Possibility of increased bleeding risk with concomitant SSRIs or SNRIs. Adverse reactions: Common: anaemia, dizziness, headache, epistaxis, abdominal pain, lower GI haemorrhage, upper GI haemorrhage, oral/pharyngeal haemorrhage, nausea, blood bilirubin increased, gamma GT increased, cutaneous soft tissue haemorrhage, rash, pruritus, macroscopic haematuria/urethral haemorrhage, vaginal haemorrhage, puncture site haemorrhage, liver function test abnormal. Serious uncommon: thrombocytopenia, hypersensitivity, intracranial haemorrhage (ICH), intraocular haemorrhage, other haemorrhage, haemoptysis, surgical site haemorrhage, Conjunctival/scleral haemorrhage, Blood alkaline phosphatase increased, Transaminases increased, Urticaria. Serious rare: anaphylactic reaction, allergic oedema, subarachnoid haemorrhage, pericardial haemorrhage, retroperitoneal haemorrhage, intramuscular haemorrhage (no compartment syndrome), intra-articular haemorrhage, subdural haemorrhage, procedural haemorrhage. Serious unknown: anticoagulant-related nephropathy. Prescribers should refer to the SmPC in relation to full side effect information. Legal category: POM Package quantities: 60mg / 30mg – 28 tablets. 15mg – 10 tablets. Marketing Authorisation (MA) number: EU/1/15/993/018, EU/1/15/993/005, EU/1/15/993/001 MA holder: Daiichi Sankyo Europe GmbH, Zielstattstrasse 48, 81379 Munich, Germany. Additional Information: Available on request from Daiichi Sankyo Ireland Ltd. Telephone: (01) 489 3000. Fax: (01) 489 3033. Email: medinfo@daiichi-sankyo.ie. Date of preparation: February 2024 IE/EDX/02/24/0002. Adverse events and product complaints should be reported. To report an adverse event or a product complaint about a Daiichi Sankyo medicine, please call Daiichi Sankyo Ireland Ltd. on (01) 4893000. Healthcare professionals can also report any suspected adverse reactions to Daiichi Sankyo medicines to the HPRA (www.hpra.ie).

Written by Dr Deirdre McDermott, Respiratory Registrar, Galway University Hospital and Dr Sinead Walsh, Consultant Respiratory Physician, Galway University Hospital & Galway City Integrated Care Hub

Asthma is one of the most common chronic respiratory diseases in Ireland, with approximately 1 in 10 people affected by this condition. Globally, asthma impacts millions of people, making it a leading cause of morbidity and healthcare utilisation worldwide. Managing asthma effectively requires a comprehensive, multi-faceted approach that engages not only healthcare providers but also patients, their families, and the wider community. In Ireland, asthma management has evolved significantly in recent years, with community-based care playing a pivotal role in improving health outcomes for individuals with asthma.
The Global Initiative for Asthma (GINA) provides guidelines that emphasise the importance of self-management education for asthma patients. According to GINA, asthma patients need comprehensive education on inhaler use, medication adherence, symptom monitoring, and the development of asthma action plans. Effective asthma management relies on empowering patients to make informed decisions about their care, including when to use medications and how to recognise when their asthma is under control or worsening.
Sláintecare is a national reform program aimed at improving healthcare access and equity in Ireland. It focuses on providing care closer to home and ensuring
Dr Deirdre McDermott
that healthcare services are more accessible to all citizens, particularly those in rural or underserved areas. Under this reform, new Regional Health Areas (RHA) have been established to offer more equitable access to healthcare services across the country. Within these RHAs, Community Healthcare Networks (CHN) have been developed. (Sláintecare - the Strategy for Improving Ireland’s Healthcare System - About the HSE, n.d.).
A key component of Sláintecare is the Integrated Care Programme for the Prevention and Management of Chronic Disease (ICPCD). This initiative aims to improve the care and management of patients living with chronic conditions, including asthma. Other chronic conditions included in the programme are COPD, diabetes and cardiovascular disease.
The ICPCD model focuses on integrating healthcare services across primary, secondary and community care, providing a patient-centred and co-ordinated approach. The model prioritises prevention, early detection, and self-management, enabling patients to receive appropriate care closer to home, reducing hospital admissions, and improving overall health outcomes. For asthma patients, this involves utilizing local services for ongoing monitoring, education, and preventative care, which greatly reduces the need for more intensive, hospitalbased interventions. (Federman et al., 2019).
One of the cornerstones of the ICPCD is the End to End Model of Care (MOC) for adults with asthma. This model of care focuses on providing comprehensive asthma
management through integrated community solutions. The MOC spans a wide range of services, from primary prevention to more specialised care. The goal is to ensure that asthma patients receive continuous, appropriate care at the most suitable level, preventing unnecessary hospitalisations and improving long-term disease control. The MOC encourages patient empowerment, ensuring that individuals with asthma are involved in decision-making processes regarding their health and treatment. This model encourages patients to manage their condition independently whenever possible, reducing the need for specialist interventions and fostering greater control over their own health.
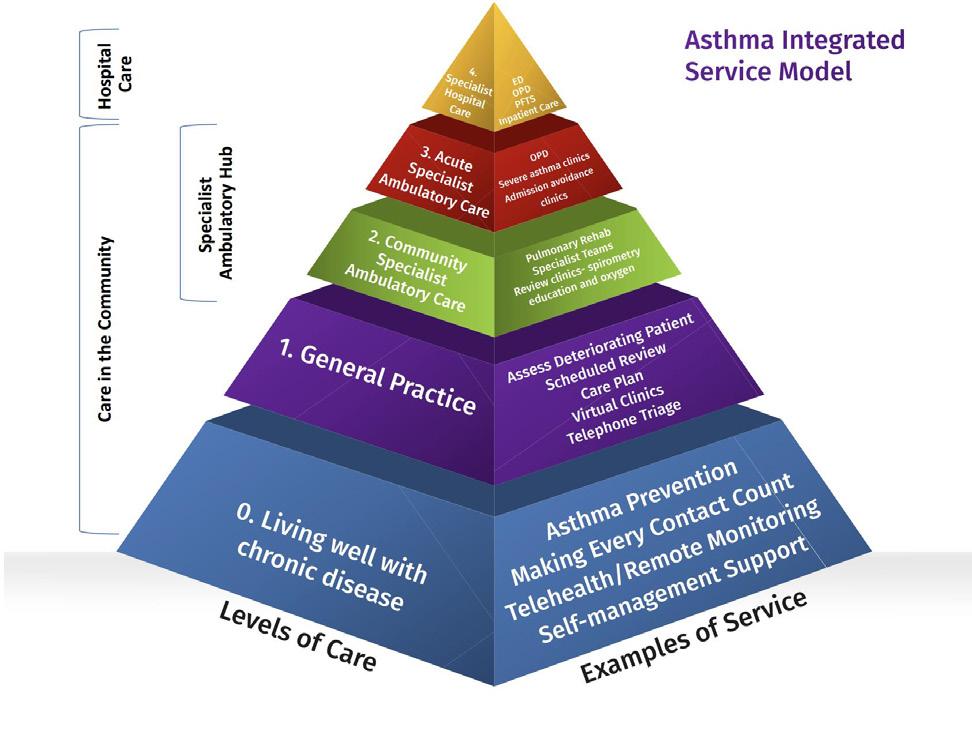
The MOC for asthma care in Ireland follows a pyramid structure, with the bulk of asthma management occurring at lower levels of care in the community setting. At Level 0, the majority of asthma patients live well with their condition and require minimal intervention. These patients are typically able to manage their symptoms with the support of general practitioners (GPs) and community healthcare services. Level 1 care involves management by GPs, who play a vital role in monitoring asthma symptoms, adjusting medications, and providing ongoing support. At Level 2, patients may be seen in Community Specialist Ambulatory Hubs, where they can access more specialised care and diagnostic services without the need to visit a hospital. Level 3 care involves acute specialty ambulatory services, where patients who require more intensive care are treated, and Level 4 care is reserved for patients who need specialty hospital care.(Model of Care - HSE.Ie, n.d.).
The pyramid approach ensures that most asthma patients are managed in community settings, with specialist interventions provided only when necessary. This structure emphasises the need for community-based care, which can prevent exacerbations and ensure that patients receive timely, appropriate care that is
closer to home. It also highlights the importance of multi-disciplinary teams (MDTs), which involve a range of healthcare professionals working together to provide comprehensive care. These teams may include GP’s, respiratory physiologists, respiratory specialists, physiotherapists, and nurses, who collaborate to deliver personalised asthma management plans, including self-management strategies and action plans. Specialist Integrated Care Hubs have been developed within the CHN’s, which offer a range of services for chronic disease management. In terms of asthma, diagnostic spirometry & FeNO testing is carried out. Consultations with asthma specialists, including the respiratory consultant and specialist respiratory nursing staff are available. After confirming the diagnosis, patients can receive educational support on their asthma self-management, including the use of peak flow meters, inhaler techniques, and the development of a personalised asthma action plan. The integrated care hubs aim to provide a “onestop-shop” for patients with asthma, where they can access both specialist care and education in a convenient, community-based setting. By bringing respiratory care out of hospital settings and into local communities, these hubs improve access to care and reduce the need for hospital visits. (Respiratory - HSE.Ie, n.d.) The importance of communitybased care in managing asthma cannot be overstated. Most asthma patients in Ireland receive their care within community settings, making it essential to ensure that these services are robust, accessible, and effectively integrated into the healthcare system. Asthma management involves ongoing monitoring, education, and personalised care, all of which can be effectively addressed within a communitybased framework. By offering regular check-ups, educational resources, and support systems, community care can significantly reduce emergency interventions and hospitalisations while improving patients’ quality of life.
A key component of communitybased asthma management is the GP Chronic Disease Management Programme. For adult patients with asthma who have a medical card or GP visit card, they can attend their GP practice to obtain a structured review of their asthma with the GP or practice nurse. The chronic disease management programme allows patients to avail of a personalised care plan, and a review of existing care plans and inhalers. If a referral to a specialist is required, the integrated care hubs are there in the community, close to where the patient lives, to provide this specialist service.
Globally, the importance of community healthcare workers in asthma management has been widely recognised. The Global Strategy for Asthma Management and Prevention underscores the role of community healthcare workers in providing education and support to asthma patients. Asthma self-management education, delivered by trained community health workers, has been shown to improve patient outcomes and reduce healthcare utilization. In many cases, community healthcare workers can deliver educational programs, monitor symptoms, and help
patients develop asthma action plans, ensuring that individuals with asthma receive the support they need to manage their condition effectively. (2024 GINA Main Report - Global Initiative for Asthma - GINA, n.d.). The success of these community-driven initiatives demonstrates the critical role of local healthcare workers in ensuring asthma patients receive the education, tools, and support necessary for managing their condition independently. This approach can reduce reliance on acute healthcare services, such as emergency department visits or hospitalisations, by promoting proactive care and selfmanagement. By teaching patients how to recognise early warning signs of asthma exacerbations, adjust their medications, and manage their symptoms effectively, community healthcare workers play a crucial role in improving asthma control.
One of the key aspects of asthma self-management is the ability to monitor asthma symptoms and peak expiratory flow (PEF). PEF monitoring allows patients to track their lung function and recognise early signs of asthma exacerbations. This empowers individuals to seek timely
medical attention and adjust their medications, helping to prevent more severe asthma episodes and reducing the need for hospital interventions. Moreover, asthma self-management education helps patients understand the importance of medication adherence, the correct use of inhalers, and avoiding triggers that can worsen asthma symptoms. Through education and ongoing support, patients can feel more confident in managing their condition independently, which leads to better asthma control and better patient wellbeing.
In both Ireland and internationally, the integration of asthma management into community care systems has been shown to improve outcomes and reduce healthcare costs. Community care not only improves access to care but also empowers patients, reduces hospitalisations, and enhances the overall quality of life for individuals with asthma.
In Ireland, initiatives such as the End to End Model of Care and Specialist Integrated Care Hubs reflect the global recognition of the importance of community-based asthma care. These initiatives align with global strategies, such as those proposed by GINA, that
emphasise the role of education, self-management, and communitybased healthcare workers in improving asthma control.
In conclusion, a communityfocused approach is central to the effective management of asthma, both in Ireland and internationally. As asthma rates continue to rise worldwide, fostering communitybased solutions will be critical to ensuring that individuals with asthma receive the support they need to manage their condition effectively. Community care is essential not only in reducing the burden on hospital systems but also in providing timely, personalised care that meets the unique needs of each patient. As the burden of asthma continues to grow, fostering community-based solutions will be essential in improving asthma care, enhancing patient outcomes, and creating a more sustainable healthcare system.
References:
2024 GINA Main Report - Global Initiative for Asthma - GINA. (n.d.). Retrieved December 13, 2024, from https://ginasthma.org/2024report/
Federman, A. D., O’Conor, R., Mindlis, I., Hoy-Rosas, J., Hauser, D., Lurio, J., Shroff, N., Lopez, R., Erblich, J., Wolf, M. S., & Wisnivesky, J. P. (2019). Effect of a Self-management Support Intervention on Asthma Outcomes in Older Adults: The SAMBA Study Randomized Clinical Trial. JAMA Internal Medicine, 179(8), 1113–1121. https://doi.org/10.1001/ JAMAINTERNMED.2019.1201
ICP for Prevention and Management of Chronic DiseaseHSE.ie. (n.d.). Retrieved December 13, 2024, from https://www.hse.ie/ eng/about/who/cspd/icp/chronicdisease/
Model of Care - HSE.ie. (n.d.). Retrieved December 13, 2024, from https://www.hse.ie/eng/ about/who/cspd/ncps/ncpr/ asthma/moc/
Respiratory - HSE.ie. (n.d.). Retrieved December 13, 2024, from https://www.hse.ie/eng/ about/who/cspd/ncps/ncpr/ Sláintecare - the strategy for improving Ireland’s healthcare system - About the HSE. (n.d.). Retrieved December 13, 2024, from https://about.hse.ie/our-work/ slaintecare-our-strategy-forimproving-irelands-healthcaresystem/

Written by Lorna Nellany, Respiratory Advanced Nurse Practitioner, Nathan Scanlon, COPD Outreach Clinical Nurse Specialist, Sligo University Hospital


Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterised by persistent airflow obstruction that is partially reversible. Clinically, it manifests as worsening dyspnoea and, in advanced stages, frequent exacerbations requiring medical intervention in primary care, emergency settings, or hospital admissions. COPD represents a major public health challenge in Ireland, significantly contributing to respiratory mortality. Despite global improvements, Ireland continues to report one of the highest agestandardised mortality rates for COPD among European nations.
The prevalence of COPD in Ireland is estimated at approximately 380,000 people. Despite this, only about 110,000 individuals have been formally diagnosed, leaving a significant proportion undiagnosed and untreated. It is a leading cause of morbidity and mortality in Ireland, with at least 1,500 deaths annually and over 15,000 hospital admissions attributed to the condition.
Sligo University Hospital (SUH) is part of the HSE West North West region, and is a public model three acute hospital with a catchment area extending to Sligo, Leitrim, South Donegal, West Cavan, as well as parts of Mayo and Roscommon. The hospital has 288 inpatient beds and provides 24/7 Emergency Medicine care, as well as various Medical and Surgical specialities
Over the years, the hospital's respiratory service has expanded from a team of one Respiratory Physician, one Clinical Nurse Specialist (CNS), and one Respiratory Physiologist to a multidisciplinary team including three Respiratory Physicians, two acute CNS, one CNS in Non-Invasive Ventilation, one CNS in COPD Outreach and one Advanced Nurse Practitioner (ANP) 50:50 Hospital and Integrated Care. There is also a Respiratory Integrated Care Team in the community comprising of two Respiratory CNS, and two Clinical Specialist Physiotherapists, two Respiratory Physiologists and a Pulmonary Rehabilitation Team.
The inpatient Respiratory CNS service plays an integral role in the care of patients with COPD. Key areas of focus include inpatient reviews, education, management of non-invasive ventilation (NIV), long term oxygen ordering and referrals to Respiratory Physiology and Pulmonary Rehabilitation. One of the most recent significant developments was the CNS team’s integration into the Emergency Department (ED) which allows the team to identify COPD patients earlier in their hospital stay, enabling timely interventions and better care coordination ensuring these patients are transferred to the Respiratory ward.
Inpatient Reviews
The respiratory CNS inpatient review service is a key component
of COPD care, offering guidance to multidisciplinary teams across various specialties. Patients are typically referred with an established diagnosis of COPD, but those who present with symptoms suggestive of COPD are also assessed then referred to the Respiratory ANP for further investigations if appropriate. The team also optimise inhaler regimens, ensuring appropriate medications, while providing education on correct inhaler techniques to improve adherence. More complex patients are discussed with the respiratory physician on consults. Through this comprehensive approach, the CNS service ensures COPD patients receive tailored, evidence-based care during their hospital stay.
Education is a cornerstone of effective COPD management, empowering patients to take control of their condition and improve their quality of life. A primary focus is on inhaler technique and the importance of medication adherence, as incorrect use of inhalers or inconsistent medication routines can significantly worsen symptoms and lead to exacerbations. Patients are also educated on the importance of winter vaccines in reducing exacerbations and maintaining lung function. Smoking cessation remains a critical area of education, as it is the most effective intervention for slowing disease progression. Additionally, patients benefit from learning to recognise the early signs of exacerbations, enabling them to seek timely intervention and avoid hospital admissions.
The CNS team plays a pivotal role in initiating and managing non-invasive ventilation (NIV) for patients with acute respiratory failure due to COPD exacerbations. We assess the patient’s clinical condition and recommend appropriate ventilator settings, including pressures and FiO2 levels, to optimise oxygenation and carbon dioxide clearance. Once NIV is initiated ongoing support and troubleshooting is provided to address challenges such as mask fit, pressure leaks, or discomfort. These patients are transferred to
the Respiratory Unit for ongoing specialist care. Ensuring effective use of NIV and addressing complications promptly significantly improves patient outcomes.
Managing patients on home oxygen therapy is another vital aspect of our role, ensuring optimal care and safety for those requiring long-term oxygen support. We work closely with our respiratory consultants to titrate oxygen, tailoring therapy to meet the patient’s needs while preventing complications such as hypercapnia. Our responsibilities also include providing phone support to patients, addressing concerns, monitoring their progress, and coordinating care. Additionally, we are often called to assess patients to determine their eligibility for home oxygen. For those who are not candidates, we evaluate alternative management plans to ensure that oxygen therapy is used effectively to enhance patient outcomes and quality of life.
The COPD Outreach service at Sligo University Hospital was established in May 2023 to address the growing burden of COPD on acute healthcare services. COPD exacerbations are a leading cause of hospital admissions and bed occupancy, yet they are recognised as an ambulatory care sensitive condition—meaning timely and appropriate community-based care can significantly reduce hospitalisations. With funding from the Enhanced Community Care (ECC) programme, the service was launched as a proactive, multidisciplinary initiative designed to improve patient outcomes, reduce healthcare utilisation and empower individuals to manage their condition more effectively.
The team consists of one Respiratory CNS and one Clinical Specialist Physiotherapist with governance from the Respiratory Consultant. National COPD Outreach guidelines provided the foundation for the programme, which were customised to meet the unique needs the catchment area.
Initially, the service began with a targeted approach, where respiratory consultants handpicked patients for inclusion. By June 2023, it expanded to include all medical patients admitted with an acute exacerbation of COPD who met the inclusion criteria. This criteria focused on patients with confirmed COPD who could engage with a self-management program and lived within a 35km radius of the hospital. Exclusion criteria included conditions such as pneumonia, pneumothorax, or social circumstances that made home care unfeasible.
The COPD Outreach service has two major streams.
Patients admitted with an acute exacerbation are followed up postdischarge for two weeks during this time they remain under the care of the respiratory consultant. They typically receive two to three home visits, depending on their clinical needs. Each visit includes:
• Monitoring of vital signs and symptoms.
• Clinical assessments, including chest auscultation and symptom questionnaires.
• Education on inhaler technique, early exacerbation recognition, and medication adherence.
• Discussions about goals of care and referrals to pulmonary rehabilitation or other services.
Patients who avoid admission during the two-week follow-up are reviewed again at six weeks, with a discharge summary sent to their GP.
In this time the COPD Outreach service demonstrated significant benefits with hospital admissions: Reduced by 78%, from 1.68 to 0.37 admissions per patient and hospital bed days reduced by 70%, from
See Figure 1

Patient Demographics
Admission avoidance targets patients who had previously engaged with COPD Outreach. Patients experiencing exacerbations at home contact the team directly for an initial phone triage. If deemed appropriate, a same or next day home visit is arranged. Nurse prescribed treatments are initiated during these visits, including oral steroids and antibiotics, often avoiding the need for hospital admission. These interventions are supported with advice from the respiratory consultant.
Both arms of the service emphasise patient empowerment, equipping individuals with the knowledge and tools to manage their disease proactively. In July 2024 we undertook an audit to measure the impact of the COPD outreach service on hospital admissions
and bed days. There were 68 accepted patients enrolled in the programme between May 2023 and March 2024, see table 1 for patient demographics of the audit carried out on 38 patients meeting pre-defined inclusion criteria.
Results and Evaluation
During this time the COPD Outreach service demonstrated significant benefits with hospital admissions: Reduced by 78%, from 1.68 to 0.37 admissions per patient and hospital bed days reduced by 70%, from 10.39 to 3.08 bed days per patient. See Figure 1.
The COPD Outreach service has demonstrated the significant impact of a tailored, communitybased approach to managing COPD. By reducing hospital admissions and bed days through proactive supported discharge and admission avoidance pathways,
the programme has not only improved patient outcomes but also alleviated the strain on hospital resources.
ANP service
The introduction of a Respiratory ANP post in 2022 led to a focus on capturing people who attended the hospital with chronic respiratory conditions but were not known to respiratory services. Currently one of the aims of the service is to reduce COPD exacerbations and ED/ hospital visits, through early diagnosis, assessment and management. This development is aligned with the Integrated Model of Care for the Prevention and Management of Chronic Disease, ensuring timely diagnosis, streamlined patient pathways, and comprehensive follow-up care.
Service implementation
The ANP-led service identifies and manages patients via two pathways
1. Emergency Department (ED) Pathway:
Patients presenting to the ED with respiratory conditions, including suspected or exacerbated COPD, are reviewed by the Respiratory CNS or ANP, a full assessment is carried out and treatment is optimised based on symptoms. If appropriate these patients are followed-up in the Respiratory ANP clinic in the Benbulbin Chronic Disease Management (CDM) Hub for further assessment, investigations and management.

COPD/ Asthma (known/suspected) ED presentation
Admitted to hospital bed
Review by Hospital consultant/ NCHD for ANP review
Review by Respiratory CNS or Respiratory Consultant & deemed suitable for ANP review community
Review by ANP Respiratory in ED/ AAU Discharge ED with follow-up
COPD with other co-morbidities
GP referral to Respiratory Consultant
Follow-up ANP clinic in the CDM hub
Follow-up review Refer Consultant clinic
Discharge GP
For patients who present Out of Hours a referral form is sent to the Respiratory ANP who contacts the patient to triage review and arrange follow-up in the Benbulbin CDM Hub. Patients are typically reviewed within 1–4 weeks of their initial presentation, gaining access to diagnostics, tailored
Past medical history
Health history
Risk factors
Smoking
Occupation
Medications
Family history
Referral for:
Pulmonary function test
FENO
Pulmonary rehab RIC
Smoking cessation
Respiratory Consultant
management plans, and selfmanagement education.
2. Inpatient Pathway:
Patients admitted to nonrespiratory specialties with COPD symptoms are flagged for review. The respiratory CNS optimises
Known to Respiratory Consultant
Triage by Respiratory Consultant
Refer ANP Consultant clinic Refer RIC
treatment during their admission, with follow-up review by the ANP in the Benbulbin CDM hub this ensures ongoing follow-up care in the community. See figure 2 for ANP referral pathway.
The ANP-led clinic operates twice weekly at the Benbulbin Chronic
Comprehensive health assessment including physical assessment
Review of available results
Reconciliation of medication
Revised treatment plan
Health promotion
Self management plan
Discharge to GP, RIC vs follow-up review with ANP. Refer to consultant clinic if complex needs – CT thorax, bronchoscopy
Disease Management (CDM) Hub, offering an efficient, “one-stop shop” approach to patient care. The patient undergoes pulmonary function testing followed by a consultation with the ANP. A comprehensive health assessment is conducted. The clinic offers ABG analysis, referral for chest
Assessment of symptoms:
SOB, cough, wheeze
Sputum
Respiratory triggers
mMRC
CAT assessment ACT
Vital signs
Sputum C&S
Bloods – IgE, RAST, Alpha 1 Antitrypsin
Allergy testing
ABG & oxygen assessment
Referral for chest x-ray

x-ray, bloods and sputum monitoring. Education on COPD management is provided, ensuring patients are informed about their condition. Treatment plans are reviewed and optimised in line with national and international COPD guidelines to ensure best practices. See Figure 3 for an overview of the clinical pathway. Most patients are discharged back to their GP with follow-up in the Chronic Disease Management Programme in primary care. This integrated approach supports early intervention, enhances patient education, and facilitates continuity of care.
Service outcomes
Between January 2023 and June 2024, 268 patients were reviewed in the ANP clinic.
COPD specific outcomes
• 93 patients with COPD were seen, of which 81 were new referrals.
• 72% of new referrals had sameday PFTs.
• 37 new diagnoses of COPD were made see figure 4
• 20 patients were referred to pulmonary rehabilitation.
• 52 patients were discharged from the ANP clinic, while 7 were referred to a respiratory consultant.
The ANP-led COPD service is closely aligned with the HSE COPD Model of Care and the Respiratory National Clinical Programme. By bridging emergency, inpatient,
and community services, this model ensures that patients receive diagnostic services, treatment plans, and access to multidisciplinary teams—all within a streamlined, patient-centred framework. This seamless integration of acute and community care improves health outcomes and supports the sustainability of respiratory services at Sligo University Hospital.
Future plans
exacerbations early and reduce hospital admissions.
We are concentrating more on treatable traits and identifying characteristics and phenotypes which respond to specific targeted treatments which means we can provide personalised precision medicine. We see the improvement in quality of life biologics have made to people with asthma and there has been exciting research on expanding the role of biologics for people who have severe COPD with asthma overlap.
disease. There is a potential to hold joint Respiratory and Cardiology clinics in the CDM hub to better manage symptoms such as breathlessness.
The ANP-led COPD service is closely aligned with the HSE COPD Model of Care and the Respiratory National Clinical Programme. By bridging emergency, inpatient, and community services, this model ensures that patients receive diagnostic services, treatment plans, and access to multidisciplinary teams all within a streamlined, patient-centred framework. This seamless integration of acute and community care improves health outcomes and supports the sustainability of respiratory services Sligo University Hospital.
There are many exciting projects happening in terms of COPD care but nationally and internationally. We would like to focus on the use of remote patient monitoring for real-time assessment of COPD exacerbations and expansion of tele-monitoring to detect
We also know that COPD rarely exists in isolation. We are beginning to appreciate that many diseases co-exist with COPD and they also interact. This is obviously the case for cardiovascular
COPD care by the Respiratory Nursing Team at Sligo University Hospital exemplifies a patientcentred, multidisciplinary approach, integrating acute and community services. From innovative inpatient reviews to the establishment of COPD Outreach and the ANP-led clinic, these services have demonstrated significant improvements in patient outcomes, resource utilisation, and quality of life.
focus on the use of remote patient monitoring for real-time assessment of
A concerned group of parents are calling for individual trials of the modulator therapies for their children with Cystic Fibrosis who still do not have access to these highly effective medications.
In 2020, the European Medicines Agency (EMA) approved the roll-out of the modulator therapy Kaftrio in Ireland by Vertex Pharmaceuticals. This authorisation covered over 900 gene variants and since then has extended to benefit more people with CF including 35 children with CF who were denied
and expansion of tele-monitoring
access in 2022. However, there remains a group of people whose CF is caused by a rare gene variant, who do not have access to these therapies.
fulfilling all research requirements for this group.
shown that Kaftrio may benefit up to 50% of pwCF who have a rare gene variant.
We are concentrating more on treatable traits and identifying characteristics and phenotypes which respond to specific targeted treatments which means we can provide personalised precision medicine. We see the improvement in quality of life biologics have made to people with asthma and there has been exciting research on expanding the role of biologics for people who have severe COPD with asthma overlap.
Currently, marketing authorisation for medications like Kaftrio is granted using genetic variant based evidence. This relies on sufficient data being gathered through clinical trials. However, for people with rare genes that cause their CF, this data does not exist as their genes are too rare. It is impossible to conduct the necessary clinical trials,
Louise Patterson from Wicklow and Abilash Kumar from Donegal are two parents of the group whose children with CF have rare genes. The group, supported by Cystic Fibrosis Ireland, believe the only solution for people with CF like their children is for the EMA to reconsider their policies and allow individual trials of the therapies, led by their clinical teams.
According to published research, real world studies in France, the United States, and Israel have
According to the group, the EMA have the power to grant their children and others in their situation a chance at improving their health, stop the irreversible damage caused by CF and offer a different future. They are calling for the children’s clinical teams to be allowed to undertake an assessment of the treatment’s efficacy for each child, and for the children to have access to these drugs if they are found to work.

Respiratory syncytial virus (RSV) is a highly contagious virus that infects the lungs and upper airways. It is transmitted by coughing, sneezing and breathing. This virus spreads every winter, with the RSV season in Ireland typically running from October to March. In healthy individuals, infection with RSV is usually self-limiting; however, RSV can cause severe infections in some people, leading to hospitalisation. Groups vulnerable to serious complications include infants, young children and older adults.
In August 2024, the Health Information and Quality Authority (HIQA) published a rapid health technology assessment (HTA) to inform an interim Government policy decision on immunisation of infants and or older adults against RSV for the 2025-2026 season.
Specifically, we looked at the oneyear costs for the Health Service Executive (HSE) of different immunisation strategies involving infants aged less than one year, and for adults aged 65 and older.
The request from the Department of Health followed a clinical recommendation from the National Immunisation Advisory Committee (NIAC) for the immunisation against RSV of all infants in their first RSV season and for adults aged 65 years or older.
In the meantime, the HSE has launched its RSV Immunisation Pathfinder Programme as a temporary measure for the current 2024-2025 season. The Pathfinder Programme applies to babies born between 1 September 2024 and 28 February 2025. Parents are encouraged to have their babies immunised free of charge before leaving the maternity unit.
Our work on this rapid HTA began in January 2024 with support from a multidisciplinary expert advisory group. Given the timeline available, the assessment comprised a description of technology, a summary of international practice, a consideration of national and international epidemiology and the burden of disease, and a costing analysis.
Strategies for the prevention of RSV in the general population typically focus on encouraging behaviours to limit virus transmission, such as reducing social contacts when an individual is symptomatic, and encouraging good hand and respiratory hygiene practices.
A number of forms of immunoprophylaxis are authorised in Europe that provide passive immunisation against RSV in children; since 2022, these now include options for infants in the general population. These include a long-acting antibody, nirsevimab (Beyfortus®), administered directly to the infant, and a maternal vaccine RSVpreF (Abrysvo®), administered to pregnant women, thereby providing infant protection through transplacental antibody transfer. At the time of our assessment, two vaccines had been authorised for the immunisation of adults aged 60 years and older: RSVPreF3 (Arexvy®), RSVpreF (Abrysvo®). Since then, a third vaccine (Respiratory Syncytial Virus mRNA
vaccine (mRESVIA®)) has also been authorised.
Prior to the 2024-2025 RSV season, which saw the introduction of the HSE’s RSV Immunisation Pathfinder Programme, palivizumab (Synagis®) was provided by the HSE for infants and children up to two years of age who were considered to be at highest risk of severe disease caused by RSV. In two published recommendations in October 2023 and April 2024, NIAC recommended that nirsevimab should replace palivizumab for these infants and children. Since the start of the 2024-2025 RSV season, the current standard practice has changed and nirsevimab is now given to these infants in place of palivizumab. Our review of international practice found that policy decisions have been informed by data from randomised controlled trials and emerging observational data from countries that implemented RSV immunisation for the 2023-2024 season. These data suggest that these technologies are safe
Over 3 RSV seasons, a single dose of AREXVY demonstrated sustained efficacy against RSVLRTD in adults aged 60 years and older 1,2
SEASON 1 OVERALL EFFICACY IN PROTECTING AGAINST RSV-LRTD3**
82.6%
SEASON 1 EFFICACY IN PROTECTING AGAINST RSV-LRTD FOR PARTICIPANTS WITH AT LEAST 1 COMORBIDITY OF INTEREST†3**
94.6%
AREXVY has a clinically acceptable safety profile1
Primary Endpoint ² 82.6% (96.95% CI*, 57.9, 94.1)
AREXVY (7 cases out of 12,466), placebo (40 cases out of 12,494)
Secondary Endpoint ² 94.6% (95% CI, 65.9, 99.9)
AREXVY (1 case out of 4937), placebo (18 cases out of 4861)
**Season 1 data with 6.7 months follow up.
Vaccination may not protect all recipients. 2
*RSV=respiratory syncytial virus; RSV-LRTD=respiratory syncytial virus-associated lower respiratory tract disease CI=Confidence Interval
†Comorbidities of interest^1: chronic obstructive pulmonary disease (COPD), asthma, any chronic respiratory/pulmonary disease, chronic heart failure, diabetes mellitus type 1 or type 2, and advanced liver or renal disease (endocrinometabolic).
Arexvypowder and suspension for suspension for injection. [Respiratory Syncytial Virus (RSV) vaccine (recombinant, adjuvanted)].
(Please refer to SmPC before prescribing).
Composition: After reconstitution, one dose (0.5 mL) contains: RSVPreF31 antigen2,3 120 micrograms. (1RSV recombinant glycoprotein F stabilised in the pre-fusion conformation = RSVPreF3, 2RSVPreF3 produced in Chinese Hamster Ovary cells by recombinant DNA technology), 3adjuvanted with AS01E containing: plant extract Quillaja saponaria Molina, fraction 21 (QS-21) 25 micrograms, 3-O-desacyl-4’-monophosphoryl lipid A (MPL) from Salmonella minnesota 25 micrograms. Therapeutic indications: Active immunization for the prevention of lower respiratory tract disease caused by RSV in adults 60 years and older and in adults 50 through 59 years of age who are at increased risk for RSV disease. The use of this vaccine should be in accordance with official recommendations. Posology and method of administration: For intramuscular injection only, preferably in the deltoid muscle. Administered as a single dose of 0.5 ml. Revaccination: need not established. Contraindications: Hypersensitivity to the active substances or any of the excipients. Special warnings and precautions for use: The name and the batch number of the administered product should be clearly recorded. Appropriate medical treatment and supervision should be readily available in case of an anaphylactic event. Vaccination should be postponed in subjects suffering from an acute severe febrile illness. However, the presence of a minor infection, such as a cold, should not result in deferral. A protective immune response may not be elicited in all vaccinees. Anxiety-related reactions, including vasovagal reactions (syncope), hyperventilation or stress-related reactions may occur. Precautions should be in place to avoid injury from fainting. Never administer intravascularly or intradermally; no data available on subcutaneous administration. Caution in individuals with thrombocytopenia or any coagulation disorder since bleeding may occur following intramuscular administration. Safety and immunogenicity data on Arexvy are not available for immunocompromised individuals. Patients with immunodeficiency or on immunosuppressive treatment may have a reduced immune response to Arexvy. Interactions: Arexvy may be administered concomitantly with inactivated seasonal influenza vaccines (standard dose unadjuvanted, high dose unadjuvanted, or standard dose adjuvanted). If Arexvy is being given at the same time as another injectable vaccine the vaccines should
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Learn more at Arexvy.ie
be administered at different sites. Fertility, pregnancy and lactation: There are no data on the effects on human fertility. Arexvy is not recommended during pregnancy or in breast-feeding/lactating women. Effects on ability to drive and use machines: Arexvy has a minor influence on the ability to drive and use machines. Undesirable effects: Very common (≥1/10): Headache, myalgia, arthralgia, injection site pain, injection site erythema, fatigue. Common (≥1/100 to <1/10): injection site swelling, fever, chills. Uncommon (≥1/1000 to <1/100): lymphadenopathy, hypersensitivity reactions, nausea, abdominal pain, vomiting, injection site pruritus, pain, malaise. Legal Category: POM A. Marketing Authorisation Number: EU/1/23/1740/001-002. Marketing Authorisation Holder: GlaxoSmithKline Biologicals S.A., Rue de l’institut 89, B-1330 Rixensart, Belgium. Further information is available from GlaxoSmithKline (Ireland) Ltd. 12 Riverwalk, Citywest Business Campus, Dublin 24. Telephone: 01-4955000. Code: PI-11947. Date of preparation: September 2024.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Please see full Prescribing Information for AREXVY, on Medicines.ie.
References: 1. Arexvy SPC, www.medicines.ie (accessed September 2024). 2. Michael G. Ison,et al., on behalf of the AReSVi-006 study group. The Efficacy of a Single Dose of the Respiratory Syncytial Virus Prefusion F Protein Vaccine in Adults ≥60 Years of Age Over 3 RSV Seasons. Poster 3391 presented at CHEST 2024 – (2024 October 6-9), Boston, United States. https://events.rdmobile.com/Lists/Details/2538335 3. Papi A, Ison MG, Langley JM, et al., for the AReSVi-006 Study Group. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595-608. doi:10.1056/ NEJMoa2209604.



authorisation of nirsevimab and the RSV vaccines, we noted that international practice is changing rapidly.
The rapid HTA considered data from the Health Protection Surveillance Centre (HPSC) and the Hospital In-Patient Enquiry (HIPE) system.
HPSC data for period 2013 and 2023 indicated that among:
• those aged 0 to 4 years, approximately two thirds of notified cases, RSV-related emergency department attendances and hospital admissions occurred in infants aged less than one year, with a disproportionate number of admissions occurring in those aged less than six months.
• adults aged 65 years and older, burden typically increased with age, with on average almost half of notified cases, RSVrelated emergency department attendances and hospital admissions reported in adults aged 80 years and older.
Hospital utilisation data from the HIPE system for the period 2013 to 2022 showed that there is a substantial burden associated with RSV in terms of hospitalisations and ICU admissions. While the annual burden has varied, the highest burden was consistently seen in those aged 0 to 4 years,
with the majority of this burden seen in those aged less than one year, and occurring in quarter four (October to December) each year.
While testing capacity has increased, we noted that the identified data are likely an underestimate of the total burden, as not all RSV cases are laboratory confirmed and some discharges may not be coded.
We undertook a costing analysis for a range of potential immunisation strategies for the 2025-2026 RSV season. Given assumptions around uptake, the cost of the products, administration costs and considering potential cost offsets due to hospitalisations averted:
• The estimated one-year cost to the HSE of immunising infants during their first RSV season ranged from ¤3.9 million to ¤19 million, depending on the approach taken. These costs would be partially offset by the fact that fewer infants would require hospital care.
• Offering an RSV vaccine to everyone aged 65 years and older was estimated to cost ¤146 million, while it would cost over ¤76 million if only offered to those aged 75 years and older. The potential for cost offsets in older adults was noted to be low as there are relatively few confirmed hospitalisations for RSV in this age group.
Moreover, we found that immunising children up to two years of age at increased risk of severe disease with nirsevimab instead of palivizumab would cost less than current care.
Our analysis found substantial uncertainty in relation to these one-year costs, and that these estimated one-year total costs were highly dependent on assuming a favourable product unit cost for the interventions.
In addition to the costs of the various strategies, we identified that there would be implementation costs (for example, IT system updates, information and training) which would likely apply with the introduction of any new RSV programme irrespective of the included target population(s) and or immunisation uptake.
We highlighted that there would be substantial organisational challenges associated with extending RSV immunisation to the general infant and or older adult population, given the large number of individuals involved and the aim to maximise uptake within a short time frame before the start of the RSV season. Specifically, this considered the potential to offer immunisation to approximately 28,000 infants born outside of the RSV season and up to 840,000 adults aged 65 years and older in primary care settings. Furthermore, given the uncertainty in relation to the cost associated with the different RSV strategies,
we advised that product cost should be a key consideration in any decision-making.
Additionally, we advised that data collection will be important to support ongoing monitoring and evaluation of the effectiveness of any programme that is implemented. A public information campaign would also be essential to raise awareness and support informed decision-making.
What’s next?
Our assessment was submitted as advice to the Minister for Health and the HSE to inform an interim policy decision on the most appropriate RSV immunisation strategy for the 2025-2026 RSV season.
We are now conducting a full HTA to provide advice to inform a longer-term government policy decision about RSV immunisation — that is, 2026 and beyond — which we will publish later this year. It will include emerging international evidence as well as experience from the HSE’s current RSV Immunisation Pathfinder Programme.
For more information:
• HIQA’s rapid HTA of immunisation against RSV is available at www.hiqa.ie.
• Information from the HSE on RSV is available at www2.hse.ie/conditions/rsv/.
• Information on the HSE’s RSV Immunisation Pathfinder Programme is available at www.gov.ie/.
A recent report by the Irish Asthma Society into the treatment of severe asthma in Ireland sheds light on the complex challenges faced by patients and the healthcare system. The comprehensive report follows an in-depth investigation into severe asthma in Ireland, which included a patient survey, focus group discussions, and a roundtable event with healthcare professionals.
Severe asthma is a chronic and potentially life-threatening condition, which significantly impacts patients' well-being, quality of life, and places a substantial economic burden on individuals and society. The delayed diagnosis and treatment of patients, the financial pressure on both a personal and state level, and the lack of awareness of severe asthma
as a distinct and debilitating disease, are just some of the challenges highlighted in the report.
Eilís Ní Chaithnía, CEO, Asthma Society of Ireland said: “The publication of this report is an important step not only in rectifying the shortcomings of severe asthma care in Ireland, but also in giving voice to people who for a long time have been suffering in silence with little recognition. Asthma can often be dismissed as a trivial illness. It is not. And particularly for patients living with severe asthma, this couldn’t be further from the truth.”
“It’s clear from the report findings that the approach to severe asthma care and support in Ireland needs close and immediate attention and we are calling on the
next Government to implement the recommendations as a matter of urgency. Furthermore, we are urging all political parties to pledge their support for a severe asthma registry, ahead of the general election”.
Approximately 450,000 people in Ireland currently have asthma, making asthma the most common chronic respiratory disease in the country. It is estimated that between 3% and 10% of that patient population has severe asthma. However, unlike many other countries, Ireland does not have a national registry of severe asthma patients, which makes it difficult to accurately assess the true number of patients.
Professor Marcus Butler, Consultant Respiratory Physician
at St Vincent's Hospital said: “We can currently only estimate the number of people in Ireland with severe asthma based on prevalence rates in other jurisdictions and on the numbers of people we treat in specialist clinics. This represents a significant challenge – it's hard to make the best policy, budgetary or service decisions if we don’t know the full extent of the situation. A severe asthma registry could provide accurate, reliable and more up-to-date information about the treated and not-yet-treated patient population and the disease. Data like this would facilitate better research, better health service planning and management and, crucially, better treatment and care for people with different forms of severe asthma.”
In the last decade, improvements in Cystic Fibrosis (CF) care have moved at a very welcome, yet rapid rate. The development of CF transmembrane conductance regulator (CFTR) modulator therapies and standardised multidisciplinary patient care have brought numerous benefits to people living with CF. Roll-out of the triple combination CFTR modulator compound, Kaftrio (Vertex pharmaceuticals), began in Ireland in September 2020.
Dubbed ‘wonder drugs’, many people with CF (pwCF) have benefited from these medications feeling less burden of disease and experiencing a new lease of life. Among the improvements are less frequent exacerbations, less hospital admissions, as well as new opportunities to travel, to work and to contemplate a future they hadn’t dared before.

CEO,
While the landscape for CF care is changing, CF remains an extraordinarily challenging condition. It must be acknowledged that there are still pwCF who are ineligible for modulator therapy, as they have a rare mutation causing their CF which has not been included in clinical trials. Others may not be able to tolerate the therapies.
For these groups, the challenges faced are as almost as historical as the condition itself - access to medication, extended hospital stays, financial constraints and, tragically, death due to CF related complications - some still as young as infants. For those with access and benefiting positively from these drugs, there are also still significant challenges which

Written by Nicola Delaney Foxe, Cystic Fibrosis Ireland
have never before been anticipated - age related conditions with CF such as menopause, the impact of co-morbidities on a person with CF, liver disease, increased cancer risks and managing a family-workCF-life balance to name but a few.
For others, the challenges of living with CF have changed, not
lessened, with the advent of the modulators. As this group moves through the natural stages of life, they are navigating fertility options, access to mortgage related products, insurance, pensions and the issues that arise when planning for a future never before considered while living with CF.
Always standing side by side with our members, Cystic Fibrosis Ireland are facing into this new era and these changing challenges with an open mind and flexibility to remain agile and respond to the needs of our membership.

Did you know, our Member Services team are always on hand to support, assist and guide members with Cystic Fibrosis who are currently staying in hospital?
Some of our services are listed below but whether it is a listening ear, signposting you to supports, or something else please feel free to contact us by calling 01 496 2433 or emailing memberservices@cfireland.ie.
A hospital admission can often present itself with additional financial costs. In efforts to support this, CFI has funds available under this scheme to assist families of children with CF and adults with CF experiencing certain financial difficulties. To help ease the financial burden, the team will work with you to help and support you where possible.
The member services team provide a listening service to all our members. During a hospital admission this can be a welcome support. Our line is open Monday to Friday, from 9am5pm where the team provide a safe environment to talk. Alternatively, you can register for a weekly call back from a member of the team during the course of your in-patient admission.
Did you know that CFI offers various financial grants to our members? For example our counselling support grant which some find beneficial during certain life stages. For more information on this and other grants please reach out to a member of the team who will be happy to help in any way they can.







Tecklenborg was announced as permanent CEO, having served as Interim CEO for most of 2024. Sarah joined CFI initially as Senior Research and Policy Coordinator bringing extensive research and management experience with her and since then, her vision has been to have the voice of the CF Community at the centre of activities.
Prior to 2024, Sarah initiated the Public and Patient Involvement (PPI) programme, support groups such as the Pregnancy Support and Advocacy Group, and since taking up the CEO position has been instrumental in campaigning for better CF care and access to medications.
Emma Wallace, CFI’s new Senior Member Services Co-ordinator came on board last June and has sought to enhance current supports and offer new services to support people to live well with CF. New services, campaigns and initiatives have since been introduced in direct response to feedback received from CFI's members.
Among these are:
1. Access to Modulators for those with rare mutations campaign A working group has been established to support the campaign for access to the modulators for the 6.7% who do not have access due to having rare mutations. CFI are calling for access to individual trials of
the modulator therapies for all those who may stand to benefit from them.
2. Access to mortgages and mortgage protection products campaign The core aim of this campaign is to address mortgage protection and its barriers for those who seek access for home ownership. The working group have also joined a subgroup with members of the Disability Federation of Ireland in efforts to collectively share knowledge and drive change in this area.
3. Inpatient care packs Another new initiative is the role out of inpatient hospital care packs to many Hospital Network Teams. Within these packs are items that may benefit patients during their stay - in addition to information detailing the services which will support them in living with CF. To support the emotional wellbeing of a patient during a hospitalisation, CFI now also offer a hospital call back service which members can register for and ensure that those who do register receive a weekly check in.
4. Parent Connect CFI, in partnership with our parents working group have formed the peer-led Parent Connect Team which aims to provide initial support and guidance to parents and create a sense of community and connection among those facing similar
challenges. As part of the service offering, the group have developed a newborn care pack for distribution to new parents at the time of diagnosis by their hospital team and run a monthly online support group for new parents to connect into.
Much done, more to do
The phrase ‘much done, more to do’ has been frequently used to describe just how far CF care has come since the foundation of CFI in 1963, but how much more work is needed. In 2025, this still rings true. Research remains a key CFI priority to further the treatment of CF and ultimately f ind a cure. Among the projects being funded by CFI is research to develop a peer connect service for young people living with CF.
However, fundamental to all the work of CFI is communication and feedback. From members, from health care professionals (HCPs), from you.
“In spite of progress challenges remain, medication shortages, access to treatments for those with rare mutations, barriers to home ownership, mortgages and pensions - all which are a focus for us in the years ahead,”
Sarah shares.
“The CF Community has always been and remains to be an incredibly strong group which has overcome many, many obstacles
A new website providing essential information about the oral health of adults and children living with cystic fibrosis (CF) has been launched.
The resource, which aims to improve dental care for people with CF, was developed following a Health Research Board funded study which explores the oral health challenges faced by individuals with cystic fibrosis.
The project was conducted at University College Cork in collaboration with Cystic Fibrosis Ireland. It had significant input from CF advocates. Dr Niamh Coffey, Senior Lecturer in RCSI's School of Dentistry, was one of the study leads.
During the project, the researchers conducted several
clinical studies, including the largest global study on this topic. Their research focused on the oral health of people with CF, the attitudes and knowledge of dental professionals, and the experiences of the CF community in accessing dental care.
Key findings from the study reveal that adults with cystic fibrosis experience more dental decay than individuals without CF. They also tend to have more developmental defects in dental enamel, which can affect the appearance of teeth and increase the risk of dental decay. Additionally, people with CF are more likely to have higher levels of dental plaque and calculus buildup.
The research also identified the potential impact of oral nutritional
in the past. Our CF medical community have always been a crucial part of the journey and CFI would like to extend our thanks and gratitude to you for all the work that you do for and with our members.”
To ensure continued communication and collaboration, CFI invite HCPs to sign up for the new CFI monthly hospital newsletter, to receive updates of our new and existing services, grants, webinars and conferences.
Teams are also invited to share activities and news with our members through our quarterly magazine Spectrum - a key channel for our members to receive updates from hospitals and clinics for over 10 years.
Sarah continues, “I am excited about the future for people with CF. I look forward to building on the progress that has been achieved through decades of hard work and advocacy and to continuing the great work of those before us. As a team, we can continue to drive change and deliver on our goal of lives free from the challenges of CF - whatever form they may take.”
To join the hospital newsletter, please email memberservices@ cfireland.ie. To contribute to Spectrum, please email ndelaneyfoxe@cfireland.ie.
To get in touch with the Parent Connect team, please email parentconnect@cfireland.ie
supplements, with many of these drinks containing high sugar levels. The study recommends that individuals using these supplements alert their dentist, allowing them to recommend additional dental products to prevent damage caused by excessive sugar content.
One particularly important finding concerns patients awaiting organ transplants. The research highlighted the need for individuals to be certified as “dentally fit” by their dentist before being placed on transplant lists, which can present a barrier to accessing timely care if dental diseases such as decay or gum disease are present.
Dr Niamh Coffey, RCSI School of Dentistry, said: “We have developed this online resource
to provide practical information about the specific oral health issues experienced by people with CF. Our goal is to empower individuals with cystic fibrosis and their families, as well as dental professionals, with the knowledge they need to better manage and maintain oral health.”
The newly launched website provides comprehensive information including oral health care tips, the relationship between transplants and dental health, the impact of reflux on teeth, inhaled steroids and their effects, and managing candida. The website also includes a special section for parents of children with CF, discussing enamel defects and the role of nutritional supplements in increasing the risk of dental decay.

Written by Carita Bramhill, Donna Langan, Helen Mulryan, Jessica Eustace-Cook, Anne-Marie Russell and Anne-Marie Brady
Interstitial lung disease (ILD) describes a range of heterogeneous lung conditions characterised by inflammation and fibrosis of the lung interstitium.1,2 In the last decade there have been significant advances in our understanding of the pathophysiology of ILDs and the introduction of treatments that have significantly changed the landscape for many patients.3 A large proportion of patients diagnosed with ILD have pulmonary fibrosis (PF)—most commonly idiopathic pulmonary fibrosis (IPF), representing around 17–37% of all ILDs.4 IPF is a chronic progressive and irreversible disease which can profoundly and devastatingly impact the physical and psychological well-being of individuals.5,6 People living with IPF may experience debilitating symptoms, which vary in severity and disease course. Symptoms include progressively worsening breathlessness, impaired lung function, cough and fatigue,7,8 with many patients and their carers experiencing anxiety and/ or depression.9,10 This high symptom burden,1,11 coupled with social isolation for some, along with an inability to perform daily activities and the adjustment to a reduced
life expectancy (median survival being 2–5 years), can impact quality of life (QoL).12
Affecting predominantly older adults13 the incidence of IPF increases with age and with higher rates seen in males.14,15 Internationally there has been a lack of standardisation in diagnostic coding, leading to an estimated reported prevalence of IPF ranging from 7 to 1650 per 100 000 persons.16 Patients living with a diagnosis of IPF have high unmet care needs and require a multi-disciplinary team approach to care which should include support such as multi-disciplinary team discussion at the time of diagnosis.1,17
Incongruence persists between the needs of patients with IPF such as accurate and timely diagnosis,18 referral for lung transplantation assessment,17,19 access to pulmonary rehabilitation9,18 and the actual delivery of healthcare services to adequately meet these needs. Individual needs of patients with IPF are important and so a person-centred approach encompassing the multiple components of the wider healthcare delivery system is needed. Addressing unmet needs of patients with IPF will contribute to improved quality of
life.18,20 Several studies including systematic reviews have previously investigated the care needs and experiences of patients with IPF.7,8,18,20–24 However, many preCOVID-19 studies have a broadly hospital-based focus with minimal recognition of the changing landscape of healthcare delivery, including community-based care. Addressing unmet needs particularly for patients with IPF is deemed to be a critical issue and may facilitate the prioritisation of health services for this patient group and ultimately lead to improved quality of life.18,20 Comprehensively understanding the unmet needs of patients with IPF can promote informed decision-making regarding patients’ ongoing care and recognition of patient preferences. Despite advancements in our understanding of the pathogenesis of the disease and the ongoing delivery of antifibrotic treatment, deficits in our understanding of the needs and research priorities of patients with IPF prevail. Addressing the unmet needs of patients with IPF and designing services and patient-centred solutions around what patients want is essential to the future development of care for patients with IPF.
This review was guided by a central question, which was to map the available evidence related to the unmet needs of patients living with a diagnosis of IPF. The central research question was developed after several meetings with patient and public partners (PPI) comprising of patients diagnosed with IPF, their carers and healthcare professionals who collectively (a) described their research priorities and (b) identified the multi-dimensional component of their unmet needs. This was an iterative process and over the course of three meetings the research question took shape and led to the development of the scoping review protocol.
Aim
This scoping literature review aimed to examine the unmet needs of patients living with a diagnosis of IPF.
Objectives
1. To synthesise the unmet needs of patients living with a diagnosis of IPF.
2. Define barriers and facilitators to meeting patients’ needs.
3. Provide an overview of relevant concepts and terminology.



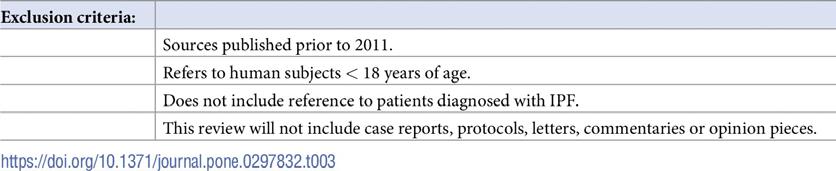
Eligibility criteria
For the purpose of this review, patient needs have been defined from (a) PPI group input and (b) built on earlier work which mapped patients’ care needs identified by the European IPF patient charter. New and emerging needs that were identified in the literature, and which to date have not been
included in the European IPF patient charter, were mapped within this scoping review.20
We utilised the ‘PCC’ framework, population (adult patients with IPF), concept (unmet needs), and context (all healthcare settings) to define the search strategy inclusion criteria.25 The PCC framework used to inform the search strategy is presented in Table 1. The ‘PCC’ mnemonic (population, concept,
and context) is recommended as a guide to construct clear objectives and eligibility criteria for scoping review.26
The core concept in this review is ‘unmet needs’. Studies describing the unmet needs specifically of patients diagnosed with IPF were included. Inclusion and exclusion criteria are presented in Tables 2 & 3.
Patient and public involvement
Patient and public involvement (PPI) is recommended from the earliest research stages through to dissemination of the study findings.27 Patient and public involvement throughout the various stages of research is a valuable component of research activity and can contribute to improved quality and relevance of research.28 Patient and public representatives (n = 5) from the Irish Lung Fibrosis Association PPI group were involved in reviewing the research protocol for this scoping review. The stakeholders comprise of patient representatives, family members of patients diagnosed with IPF
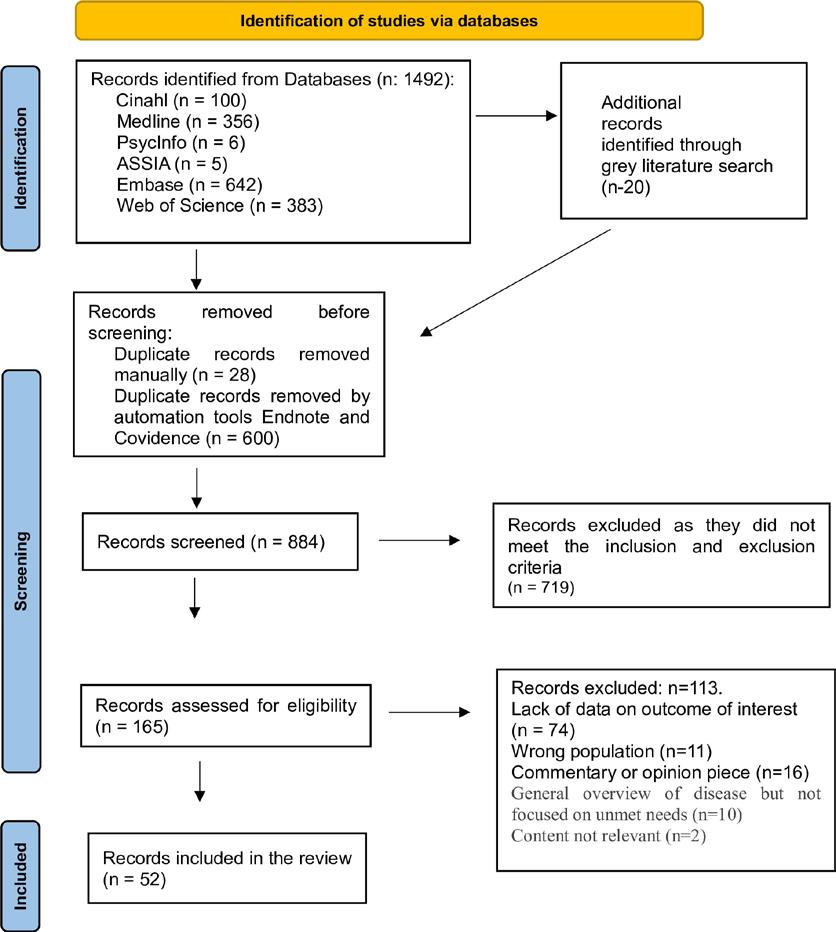
and experts and researchers in the field of IPF. The scoping review protocol was presented to the group for feedback and discussion during a Zoom call meeting. The scoping review findings were also presented to healthcare professionals in the field of IPF in person for further opportunities for discussion. The involvement of PPI in this scoping review has enriched the review and reflected the importance of including those impacted by IPF in the process.
Sources & searching
The search strategy was developed with the assistance of a medical research librarian (JEC) and externally peer-reviewed by a second librarian as per the Peer Review of Electronic Search Strategies (PRESS) guidelines.31 Six databases were systematically searched including, Medline, CINAHL, APA PsycINFO, (EBSCO platform), Embase (Elsevier), and Web of Science (Core Collection) and ASSIA (Applied Social Science Index) (Proquest), on 14th November 2022 and updated on 12th January 2023 to identify studies that met the review’s inclusion criteria. Date limit criteria was applied at full text review (2011-present). A restriction to literature published pre-2011 was applied as this was around the time that antifibrotic treatment for patients with IPF became available in the United Kingdom and Europe. No language or geographic limits were applied. Grey literature and unpublished studies were included; sources included ProQuest Dissertations and Thesis Global, Google Scholar and ClinicalTrials.gov, WHO International, Clinical Trials Registry Platform and OpenGrey. Further, a comprehensive online search of key websites and a
manual search of the reference lists of included studies were performed. Several international conference abstracts were reviewed including those from the Irish Thoracic Society annual scientific meeting, the European Respiratory Society annual meeting, the British thoracic society meetings, and the American Thoracic Society meeting. A total of 100 abstracts were reviewed with 9 abstracts included in the final review.
The search strategy and database search were both conceptualised by the researcher (CB) and an information specialist (JEC).
Key search terms related to ‘IPF’, ‘unmet needs’, ‘idiopathic pulmonary fibrosis’ and ‘pulmonary fibrosis’ (The search string is available in S3 Table).
An example of a search completed on CINAHL (EBSCO) of the search terms to identify the population is included here using the ‘PCC’ acronym and specifically looking at the ‘population’ component of PCC, (MH "Idiopathic Pulmonary Fibrosis") OR (MH "Idiopathic Interstitial Pneumonias+") OR (MH "Pulmonary Fibrosis+") OR TI (“Idiopathic pulmonary fibros*” OR “Idiopathic interstitial pneumonia*” OR “Familial Idiopathic Pulmonary Fibrosis*” OR “Usual Interstitial Pneumon*” OR “fibrosing interstitial lung disease” OR “progressive fibrosis” OR “nonspecific interstitial pneumonia” OR “pulmonary fibros*”) OR AB (“Idiopathic pulmonary fibros*” OR “Idiopathic interstitial pneumonia*” OR “Familial Idiopathic Pulmonary Fibrosis*” OR “Usual Interstitial Pneumon*” OR “fibrosing interstitial lung disease” OR “progressive fibrosis” OR “nonspecific interstitial pneumonia” OR “pulmonary fibros*”).

Initially, all identified records were collated and uploaded onto EndNote X9.3.3 (Clarivate Analytics, Pennsylvania, USA) and duplicates removed. Then all identified citations were transferred into Covidence software (Veritas Health Innovation, Melbourne, Australia) where any remaining duplicates were removed.
The next step was to screen the titles and abstracts conducted by two independent reviewers (CB and DL) for assessment against the inclusion criteria. Potentially relevant studies which met the inclusion criteria were retrieved in full text and uploaded to Covidence. The full text of selected citations was assessed in detail against the inclusion criteria (PCC inclusion criteria) by the same two independent reviewers. Any disagreements which occurred about the inclusion or exclusion of a paper were discussed by the reviewers until agreement was found. A third reviewer HM arbitrated when there was disagreement about the inclusion of a paper (n-4).
We included all review types (systematic, scoping and narrative reviews) which described the unmet needs of our patient group. This review did not include case reports, protocols, letters, commentaries or opinion pieces.
Data analysis
Data were analysed utilizing the Braun and Clarke (2006) framework for reflexive thematic analysis (TA).32 This is a fivestage process for coding and data analysis and includes an initial step of data familiarisation through deep immersion with the information sources. Next, an iterative process of code development was supported by
version 12.0). The final three steps provided the backdrop to the organisation of codes into themes and fostered a rich in-depth analysis of the data, culminating in theme development. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) displays the identified sources (Fig 1).
Summary of the studies
A total of 1492 information sources were identified through our database search, and a further 20 records identified through a grey literature search, with 628 duplicates removed through manual and automated tools. After eliminating duplications, 884 unique citations were identified with five of these representing non-English sources. Of these, 719 records were excluded after title/ abstract screening, leaving 165 records for further assessment. After a full text review, 113 records were deemed ineligible and were excluded. The primary reason for exclusion was the lack of data on the outcome of interest (n = 74). Other common reasons for exclusion were the wrong population (n = 11) and commentary or opinion piece (n = 16). Fifty-two information sources met the inclusion criteria, of which (n = 30/58%) were published in the last five years. All included articles were published between the period of 2011 to 2022. Included sources were published in English and were from a wide geographical area, including several countries where English is not the dominant spoken language and presented in Fig 2.
There was a total of thirty-eight studies included in this review, representing 73% of included
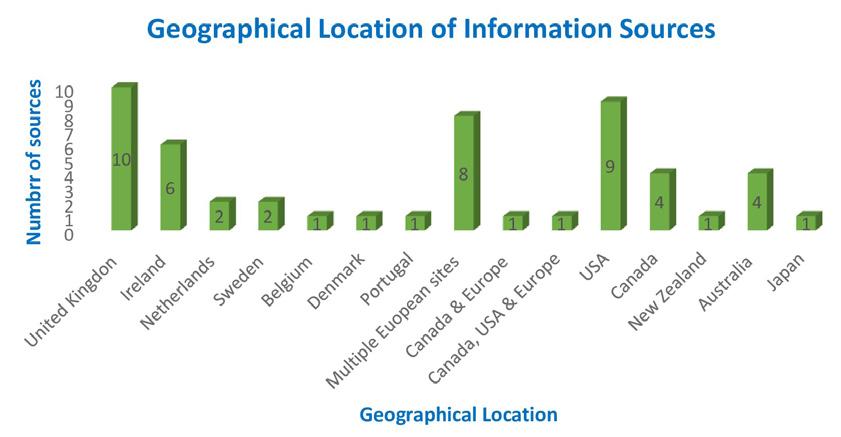

records, with the remaining (n = 14/27%) records being a mix of literature reviews (n = 6), reports and guideline-type material, incorporating various methods (n = 8).
Of the studies (n = 38/73%), 50% of them employed quantitative approaches (n = 19), with others using a qualitative design, 37% (n = 14), while further studies employed a mixed method methodology 10.5% (n = 4). Several literature reviews (n = 6/11.5%) were included in the overall review of fifty-two records and incorporated a range of methods. The remaining information sources (n = 8/15.3%) were diverse and included guideline documents (n = 2), patient charters (n = 2), framework documents (n = 1) reports (n = 2) and a position statement (n = 1).
Of the thirty-eight studies (73%) included in the review, >31.6% (n = 12) of these investigated care experiences whilst a further 65.7% (n = 25) explored palliative care needs and advanced care planning. The remaining key areas of focus included in the thirty-eight studies predominantly explored information needs 71% (n = 27), pharmaceutical treatments 65.8% (n = 25), early and accurate diagnosis 47.3% (n = 18) and psychological and emotional needs 52.6% (n = 20). Other significant areas included the impact on relationships 21.1% (n = 8), physical burden of IPF 36.8% (n = 14), oxygen needs 31.6% (n = 12), pulmonary rehabilitation 36.8% (n = 14), carer experience 39.5% (n = 15), geographical location 15.8% (n = 6), access to a tertiary centre 23.7% (n = 9), multi-disciplinary team 15.8% (n = 6), ILD nurse 23.7% (n = 9), tele-health 10.5% (n = 4), lung transplantation 31.6% (n = 12) and clinical trial access 7.9% (n = 3).
Recruitment strategies within the literature were reported to be via single sites 39.4% (n = 15) or multiple ones ranging from two to fourteen sites 60.5% (n = 23). A homogenous IPF sample was investigated in most sources (84%). Remaining studies featured heterogenous samples and included other ILD’s, specifically fibrosing interstitial lung disease (F-ILD) (5.2%), pulmonary fibrosis (8.2%) and ILD (2.6%).
Male participants were significantly more frequently represented in all included studies (range 41.3%-87.7%). The fact that males were more frequently represented in our selected studies is aligned with international evidence that IPF is a disease
predominantly seen in males (30) accounting for ~70% of all IPF cases in international cohorts, which may in part be explained by the fact that men are more likely to present with a smoking history and having experienced occupational exposures more frequently than women with IPF. (31) Study participants had an age range of 20–90 years. Studies which reported ethnicity or race of participants included white backgrounds (n = 5/13.1%).
The psychological and emotional impact of an IPF diagnosis
A need exists for psychological and emotional support throughout the disease course for patients diagnosed with IPF.18,50 The literature reports that the psychosocial needs of patients41 and their family carers are frequently being overlooked,7,34 with a continued lack of psychological supports for patients diagnosed with IPF.9,34 Psychological distress is reported by many patients living with IPF, including worry, fear, anxiety, hopelessness and helplessness.7,12,23,34 It is reported that many patients with IPF and their carers experience anxiety and or depression.9,23,34,74
Patients diagnosed with IPF report a loss of independence coupled with feelings of powerlessness and social isolation.23 The initiation of oxygen therapy is viewed as a particularly stressful time for patients,23 representing for some a distressing trajectory in the disease course and in some cases a loss of hope.34 In one study oxygen initiation was associated with feelings of shame,41 as the condition became externally visible to others.34 Patients can also experience increased anxiety related to worry associated with having adequate supplies of prescribed oxygen.41
Glaspole and colleagues explored the frequency of prolonged anxiety and depression among people living with IPF and factors contributing to their persistence. They reported that dyspnoea is a major contributor to anxiety and depression followed by cough, which is also an important contributor.75 Moor et al. found that although patients were not being specifically asked about access to psychological support in their study, 10% of patients spontaneously reported a need for improved psychological support throughout the disease course.18 Van Manen and colleagues highlight the important role an ILD specialist nurse can play in helping patients to manage symptoms such as depression and anxiety,
particularly as nurses will most likely have been involved in the patients’ care for some time and may be viewed by patients as someone, they can confide in.56
High symptom burden
A significant unmet need related to IPF is the burden associated with the physical and psychological impact of the condition. IPF remains an unpredictable disease of variable course which could benefit from a systems approach to care, coordinated by a multi-disciplinary team.6 The deterioration in health-related quality of life for patients with IPF is highly correlated to worsening of symptoms, including increased breathlessness, cough and fatigue over time.12 Lindell et al. analysed focus group data and highlighted that symptoms introduce an overwhelming burden for both the patient and carer, with cough being a particularly challenging symptom.7 For some patients coughing led to distressing symptoms such as incontinence. [66] Patients report struggling with lethargy which can impact even the simplest of tasks, such as reading or watching television.22
Several patients in Duck’s study reported feeling depressed which was associated with a lack of control and having to relinquish roles once held.22 There is growing evidence that daily activities, recreation, pleasure and employment are significantly affected by the burden of symptoms like anxiety, depression and social isolation that are connected with a diagnosis of IPF.12 There were also reported symptoms from the side effects of medication—in particular antifibrotic treatment adding to the burden of symptoms already experienced by patients.43
Sampson and colleagues call for a more pragmatic needs assessment to include components of physical and social functioning, nutrition and symptom burden which would support patients’ selfmanagement and assist with their understanding of the illness and its varied disease trajectories.6
Discussion
This scoping review characterised the broad and varied unmet needs of patients living with a diagnosis of IPF. These unmet needs spanned five core domains incorporating physical and psychological needs, palliative care needs and finally needs related to information and health service provision.
In recent years our knowledge of the pathogenesis of IPF, coupled with an improved awareness of the complex disease burden associated with this disease, has rapidly improved; nevertheless, there remains a myriad of unmet needs and gaps in care for this patient population, which is reflected in several previous studies on the topic.6,18,20
Despite advancements in drug therapies including the use of antifibrotic medication, which when first introduced heralded improved survival rates for many, there continues to be unmet needs and compromised quality of life for many patients living with IPF.21 A key element of the inequalities and gaps in care that continue to exist for many patients is timely access to these antifibrotic medications87 with continued barriers to treatment such as reimbursement restrictions evidenced internationally, despite the various calls which support early initiation of these treatments.18
Conclusion
In recent years we have seen significant advances in relation to our understanding of the pathogenesis of IPF, coupled with the introduction of antifibrotic medication and their recognised contribution to patient survival. The concept of unmet needs and quality of life are intrinsically linked and yet there remains deficits in the literature as regards comprehensively investigating this relationship for patients with IPF, representing a need for future research focus examining this relationship.
This review will extend the knowledge base of the mutidisciplinary team as regards the diverse range of needs that patient with IPF have and signals the need to continue to target research toward this underrepresented patient population. The literature highlights the continued lack of integrated clinical care programmes in many jurisdictions for the management of IPF, which can result in unstructured and fragmented care delivery for patients. This study also highlights that patients living with a diagnosis of IPF experience a diverse scope of unmet needs across a broad range of areas and require a comprehensive multi-disciplinary approach to care, with equal access to services and tailored information to support them over the course of the disease. These are key areas for future research. References available on request

Fingolimod Teva 0.5 mg hard capsules
Fingolimod Teva is indicated as single disease modifying therapy in highly active relapsing remitting multiple sclerosis for the following groups of adult patients and paediatric patients aged 10 years and older:
Patients with highly active disease despite a full and adequate course of treatment with at least one disease modifying therapy (for exceptions and information about washout periods see the SmPC) or
Patients with rapidly evolving severe relapsing remitting multiple sclerosis defined by 2 or more disabling relapses in one year, and with 1 or more Gadolinium enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI.
Fingolimod Teva Hard Capsules Abbreviated Prescribing Information
Presentation: Each hard capsule contains 0.5mg fingolimod as hydrochloride.
Indications: Fingolimod Teva is indicated as single disease modifying therapy in highly active relapsing remitting multiple sclerosis (MS) for adult patients and paediatric patients (10 years and older) with: Highly active disease despite a full and adequate course of treatment with at least one disease modifying therapy; Or with rapidly evolving severe relapsing remitting MS defined by 2 or more disabling relapses in one year, and with 1 or more Gadolinium enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI. Dosage and administration: For oral administration. Treatment should be initiated and supervised by a physician experienced in MS. Adults: The recommended dose is one 0.5mg capsule taken orally once daily. Children (10 years of age and above): The recommended dose is dependent on body weight. Paediatric patients with body weight ≤40kg: one 0.25mg capsule taken orally once daily. Paediatric patients with body weight >40kg: one 0.5mg capsule taken orally once daily. Paediatric patients who start on 0.25mg capsules and subsequently reach a stable body weight above 40kg should be switched to 0.5mg capsules. The safety and efficacy of fingolimod in children aged below 10 years has not been established. Elderly: Fingolimod Teva should be used with caution in patients aged 65 years and over due to insufficient data on safety and efficacy. Renal impairment: No dose adjustments are needed in patients with mild to severe renal impairment. Hepatic impairment: No dose adjustment needed in patients with mild or moderate hepatic impairment, but caution should be exercised when initiating treatment in these patients. Fingolimod Teva must not be used in patients with severe hepatic impairment (Child-Pugh class C). Contraindications: Immunodeficiency syndrome. Patients with increased risk for opportunistic infections, including immunocompromised patients (including those currently receiving immunosuppressive therapies or those immunocompromised by prior therapies). Severe active infections and active chronic infections (hepatitis, tuberculosis). Active malignancies. Severe liver impairment (Child-Pugh class C). Patients who in the previous 6 months had myocardial infarction (MI), unstable angina pectoris, stroke/transient ischaemic attack (TIA), decompensated heart failure (requiring inpatient treatment), or New York Heart Association (NYHA) class III/IV heart failure. Patients with severe cardiac arrhythmias requiring anti-arrhythmic treatment with class Ia or class III anti-arrhythmic medicinal products. Patients with second-degree Mobitz type II atrioventricular (AV) block or third-degree AV block, or sick-sinus syndrome, if they do not wear a pacemaker. Patients with a baseline QTc interval ≥ 500 msec. During pregnancy and in women of childbearing potential not using effective contraception. Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: Initiation of treatment results in a transient decrease in heart rate (HR) and may also be associated with AV conduction delays, including the occurrence of isolated reports of transient, spontaneously resolving complete AV block. All patients should have an electrocardiogram (ECG) and blood pressure measurement performed prior to and 6 hours after the first dose of Fingolimod Teva. All patients should be monitored for a period of 6 hours for signs and symptoms of bradycardia with hourly HR and blood pressure measurement. Continuous ECG monitoring during this 6 hour period is recommended. The same precautions as for the first dose are recommended when patients are switched from the 0.25mg to the 0.5mg daily dose. In the event of post-dose bradyarrhythmia-related symptoms, initiate clinical management and monitor the patient until the symptoms have resolved. Very rare cases of T-wave inversion have been reported in adult patients treated with fingolimod. In case of T-wave inversion, the prescriber should ensure that there are no associated myocardial ischaemia signs or symptoms. If myocardial ischaemia is suspected, seek advice from a cardiologist. Fingolimod Teva should not be used in patients with sino-atrial heart block, a history of symptomatic bradycardia, recurrent syncope or cardiac arrest, or in patients with significant QT prolongation, uncontrolled hypertension or severe sleep apnoea. Fingolimod has an immunosuppressive effect that predisposes patients to an infection risk, including opportunistic infections that can be fatal, and increases the risk of developing lymphomas and other malignancies, particularly those of the skin. Before initiating treatment with Fingolimod Teva, a recent complete blood count (CBC) (i.e.
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie
Prescription Only Medicine.
within 6 months or after discontinuation of prior therapy) should be available.
Assessments of CBC are also recommended periodically during treatment, at month 3 and at least yearly thereafter, and in case of signs of infection. Absolute lymphocyte count <0.2x109/l, if confirmed, should lead to treatment interruption until recovery. Initiation of treatment with Fingolimod Teva should be delayed in patients with severe active infection until resolution. Suspension of Fingolimod Teva should be considered if a patient develops a serious infection and consideration of benefit-risk should be undertaken prior to re-initiation of therapy. Patients should report symptoms of infection up to 2 months after discontinuation of fingolimod. Serious, life-threatening, and sometimes fatal cases of encephalitis, meningitis or meningoencephalitis caused by herpes simplex and varicella zoster viruses have occurred with Fingolimod Teva. Treatment should be stopped if these develop, and appropriate treatment initiated. Patients need to be assessed for their immunity to varicella (chickenpox) prior to treatment. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment. Cases of cryptococcal meningitis, sometimes fatal, have been reported after approximately 2-3 years of treatment. If cryptococcal meningitis is diagnosed, fingolimod should be suspended and appropriate treatment should be initiated. Progressive multifocal leukoencephalopathy (PML) has been reported under fingolimod treatment. Before initiating treatment with fingolimod, a baseline MRI should be available (usually within 3 months) as a reference. MRI findings may be apparent before clinical signs or symptoms. If PML is suspected, MRI should be performed immediately for diagnostic purposes and treatment with fingolimod should be suspended until PML has been excluded. Due to the immunosuppressive properties of fingolimod, vaccination against Human papilloma virus (HPV) should be considered prior to treatment initiation. Macular oedema has been reported in patients treated with fingolimod 0.5mg. Perform an ophthalmological evaluation 3–4 months after fingolimod initiation. Evaluate the fundus, including the macula, in patients reporting visual disturbances. Fingolimod Teva should be discontinued if a patient develops macular oedema. Increased hepatic enzymes have been reported in MS patients treated with fingolimod. Signs of liver injury, including markedly elevated serum hepatic enzymes and elevated total bilirubin, have occurred as early as ten days after the first dose and have also been reported after prolonged use. Do not use fingolimod in patients with severe pre-existing hepatic injury (Child-Pugh class C). Due to the immunosuppressive properties of fingolimod, initiation of treatment should be delayed in patients with active viral hepatitis until resolution. Recent (i.e. within last 6 months) transaminase and bilirubin levels should be available before initiation of treatment. If liver transaminases are at least 5 times the upper limit of normal (ULN) or at least 3 times the ULN associated with any increase in serum bilirubin, Fingolimod Teva should be discontinued. Blood pressure should be regularly monitored during treatment as Fingolimod Teva can cause hypertension. Fingolimod Teva should be used with caution in patients with severe respiratory disease, pulmonary fibrosis and chronic obstructive pulmonary disease. Rare cases of posterior reversible encephalopathy syndrome (PRES) have been reported during treatment. If PRES is suspected, Fingolimod Teva should be discontinued. When switching patients from another disease modifying therapy to Fingolimod Teva, a CBC is recommended prior to initiating treatment to ensure that immune effects of the previous therapy have resolved. Vigilance for skin lesions is warranted and a medical evaluation of the skin is recommended at initiation, and then every 6 to 12 months taking into consideration clinical judgement. Patients should be referred to a dermatologist in case suspicious lesions are detected. Patients treated with fingolimod should be cautioned against exposure to sunlight without protection. If lymphoma is suspected during treatment, Fingolimod Teva should be discontinued. Rare cases of tumefactive lesions associated with MS relapse have been reported. Severe exacerbation of disease has been observed rarely in some patients stopping fingolimod. Caution is therefore indicated when stopping fingolimod therapy. If a decision is made to stop treatment with fingolimod a 6-week interval without therapy is needed, based on half-life, to clear fingolimod from the circulation. Caution is indicated with the use of immunosuppressants soon after the discontinuation due to possible additive immune system effects. The safety profile in paediatric patients is
similar to that in adults. Cases of seizures, anxiety, depressed mood and depression have been reported with a higher incidence in paediatric patients treated with fingolimod compared to patients treated with interferon beta-1a. It is recommended that paediatric patients complete all immunisations in accordance with current immunisation guidelines before starting Fingolimod Teva. Interactions: Anti-neoplastic, immunomodulatory or immunosuppressive therapies should not be co-administered due to the risk of additive immune system effects. During and for up to two months after treatment vaccination may be less effective. The use of live attenuated vaccines may carry a risk of infections and should be avoided. Treatment with Fingolimod Teva should not be initiated in patients receiving beta-blockers, or other substances which may decrease HR, such as class Ia and III antiarrhythmics, calcium channel blockers (such as verapamil or diltiazem), ivabradine, digoxin, anticholinesteratic agents or pilocarpine. Co-administration of fingolimod with ketoconazole resulted in a 1.7-fold increase in fingolimod and fingolimod phosphate exposure (AUC) by inhibition of CYP4F2. Caution should be exercised with substances that may inhibit CYP3A4 (protease inhibitors, azole antifungals and some macrolides such as clarithromycin or telithromycin). Co-administration of strong CYP3A4 inducers (rifampicin, phenobarbital, phenytoin, efavirenz) should be used with caution as they may reduce the AUC or fingolimod and its metabolite. Concomitant administration with St. John’s Wort is not recommended. Co-administration of fingolimod with oral contraceptives (ethinylestradiol and levonorgestrel) did not elicit any change in oral contraceptive exposure. Pregnancy and lactation: Fingolimod is contraindicated in women of childbearing potential not using effective contraception. Post-marketing data suggest that use of fingolimod is associated with a 2-fold increased risk of major congenital malformations when administered during pregnancy compared with the rate observed in the general population. Women of childbearing potential should provide a negative pregnancy test result before treatment initiation and must receive counselling regarding the serious risk to the foetus. Effective contraception must be used during treatment and for 2 months after discontinuation of Fingolimod Teva. Fingolimod should be stopped 2 months before planning a pregnancy. If a woman becomes pregnant during treatment, fingolimod must be discontinued. When stopping fingolimod therapy for planning a pregnancy the possible return of disease activity should be considered. Women receiving Fingolimod Teva should not breastfeed. Effects on ability to drive and use machines: Has no or negligible influence on the ability to drive and use machines, however, dizziness or drowsiness may occasionally occur when initiating treatment. Adverse reactions: Pneumonia, progressive multifocal leukoencephalopathy (PML), cryptococcal infections, basal cell carcinoma, malignant melanoma, lymphoma, squamous cell carcinoma, Kaposi’s sarcoma, leucopenia, thrombocytopenia, autoimmune haemolytic anaemia, hypersensitivity reactions, seizure, posterior reversible encephalopathy syndrome (PRES), macular oedema, bradycardia, ECG T-wave inversion and acute hepatic failure. Very Common: Influenza, sinusitis, headache, cough, diarrhoea, back pain and increased hepatic enzyme levels. Common: Herpes viral infections, bronchitis, tinea versicolor, lymphopenia, depression, dizziness, migraine, blurred vision, AV block, hypertension, dyspnoea, eczema, alopecia, pruritus, myalgia, arthralgia, asthenia, decreased weight and increased blood triglyceride levels. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: If an overdose constitutes first exposure to fingolimod, it is important to monitor patients with a continuous (real time) ECG and hourly measurement of HR and blood pressure, at least during the first 6 hours. If after 6 hours the HR is <45bpm in adults, <55bpm in paediatric patients aged 12 years and above, or <60bpm in paediatric patients aged 10 years to below 12 years, or if the ECG at 6 hours after the first dose shows second degree or higher AV block, or if it shows a QTc interval ≥500msec, monitoring should be extended at least for overnight and until the findings have resolved. The occurrence at any time of third degree AV block should also lead to extended monitoring including overnight monitoring. Neither dialysis nor plasma exchange results in removal of fingolimod from the body. Legal category: POM Marketing Authorisation Number: PA0436/047/001. Marketing Authorisation Holder: Norton Waterford, T/A IVAX Pharmaceuticals Ireland, Unit 301, IDA Industrial Park, Cork Road, Waterford, Ireland Job Code: MED-IE-00050.
Further information is available on request or in the SmPC. Product Information also available on the HPRA website. Date of Preparation: September 2024 | Job Code: FIN-IE-00002 Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com

Exploring patient perspectives of barriers and facilitators to participating in hospital-based pulmonary rehabilitation in patients diagnosed with non-small-cell lung cancer treated with curative intent
Written by Denise Doyle1, Matthew O’Brien2, Rosemary Murphy2
,
Aidan O’Brien
1,2,
Deirdre McGrath1,2 & Dervla Kelly
1
1Faculty of Education and Health Services, School of Medicine, University of Limerick
2University Hospital Limerick, Limerick

Lung cancer is the third most common cancer diagnosed in Ireland with non-small-cell lung cancer (NSCLC) being the most common histopathological subtype (National Cancer Registry, 2022). Surgical resection remains the gold standard treatment in early NSCLC;1 however, the surgery itself can cause significant pulmonary function impairment.2
Pulmonary rehabilitation (PR) plays an important part in recovery from treatment postlung resection surgery. PR is a comprehensive programme which amalgamates exercise training with psychological and behavioural tools to rebuild the functional capacity of patients.3 Several studies have demonstrated the safety and efficacy of PR whilst
documenting improvements in exercise capacity, muscle strength, dyspnoea, fatigue, and health-related quality of life post-lung resection surgery.4,5,6,7 Despite a wealth of evidence demonstrating the effectiveness of PR post-lung resection surgery, implementation in clinical practice remains suboptimal. Furthermore, programme implementation
in small, local cancer centres has been under researched to date. Research highlights that the successful implementation of evidence into practice requires tailoring of rehabilitation programmes to a specific setting with local stakeholders so that barriers to implementation in the local context can be understood and addressed.8, 9
While previous research has highlighted some barriers to implementation of lung cancer rehabilitation interventions4, 10, 11 the main challenge in this area today is to get stakeholder input and design and tailor strategies that address these barriers. Taking a participatory health research approach to implementation problems has the potential to improve the uptake, adherence, and sustainability of healthcare interventions.8, 9
Participatory health research strives to maximise the participation of end-users in all stages of the research process. Research is not done “on” people as passive subjects providing “data” but “with” them to provide relevant information for improving their lives.12 Applying these methodologies to pulmonary rehabilitation in cancer could provide deeper understanding of what makes these programmes work.
Despite a favourable prognosis and extensive evidence suggesting the potential positive effects of PR, patients with NSCLC still suffer from unmet rehabilitation needs. Designing and implementing a successful PR programme on a local level is logistically challenging and involves an extensive multidisciplinary team. This study aims to explore the potential barriers and facilitators to the implementation of such programmes and to identify strategies to successful operationalisation of PR programmes for those who have undergone curative resection of NSCLC.
Study design
A qualitative study using semistructured focus groups with patients who had undergone resection for NSCLC was performed. A total of 12 patients were interviewed for this study, all of whom were offered PR with no obligation to partake. Eight of the study participants opted to partake in the PR programme offered in a physiotherapy gym located on the grounds of an acute hospital.
Throughout this research endeavour, the COnsolidated criteria for REporting Qualitative research (COREQ) were used to perform and report this study.13 Furthermore, we have employed normalisation process theory
(NPT) as a framework to assess the descriptions of exercise behaviours and healthcare practices from the people recovering from lung cancer and health professionals in order to identify facilitators and barriers to rehabilitation exercise, the environment (hospital), and if possible, any strategies that may help with implementation. Developed by May and Finch, NPT is a framework that can be applied across healthcare contexts and topics to explain, and potentially shape, implementation processes.14 NPT has four components that provide a framework for this process. By incorporating theory, we hope this can help us overcome barriers and enhance facilitators and evaluate the implementation in the future.
Having gained ethical approval, patients with a diagnosis of NSCLC who had undergone lung resection were identified. Patients attending routine follow-up appointments during the period of the study (September–December 2022) were invited to participate by an administrative gatekeeper not directly involved in their care. The study inclusion criteria were those aged 18 years or over with a diagnosis of lung cancer who were treated with surgery between the period of 1st January 2019 and 31st December 2020. Twelve participants volunteered to take part in 3 focus groups. Recruitment was stopped after the third focus group as there were no new themes being identified in the interviews. No repeat interviews were performed. Of the 12 focus group participants, 8 opted to partake in the PR programme. Participation was voluntary and no disadvantages arose from non-participation. Participants were supplied with information on the objectives of the focus group interviews, and written informed consent was obtained from all participants.
The gym is an outpatient rehabilitation gym located on the site of University Hospital Limerick, Ireland. The physiotherapists provided goal-directed pulmonary rehabilitation to individuals who had undergone surgery for NSCLC. In line with infection control policy, groups of 6 individuals were scheduled for rehabilitation sessions to enable

adequate social distancing and all participants were required to wear masks. All participants were screened prior to and after each session for wellness, suitability to partake in exercise and BP, SpO2, and HR were recorded pre- and post-sessions. Participants were also provided with additional education sessions along with the option to avail of counselling. From here, individuals are discharged home to continue with exercise as they see fit. An image of the rehabilitation gym and a list of equipment available is provided in Appendix A.
The multidisciplinary research team consisted of 6 researchers. Two consultant physicians, one pharmacist, one medical student, one physiotherapist, and one advanced nurse practitioner. Relationships were not established between the participants and researchers prior to the study. Brief introductions were had prior to the commencement of the focus groups to provide some information about the study and its potential usefulness.
Data collection
Focus groups were conducted by 4 members of the research team. Focus groups were conducted between November and December 2022 with a total sample size of N = 12. Three of the focus groups were conducted in person and one via Zoom. The interviews lasted approximately one hour. A critical review of available literature on PR after lung cancer surgery was conducted to gain an adequate understanding of the subject, and from this, an appropriate list of questions was compiled to ask participants during each interview. All of the focus groups were digitally recorded and transcribed verbatim by a member of the research team. The transcripts were pseudonymized and given a consecutive ID number from 1 to 12. Transcripts were not returned to participants for comment and/or correction.
Four anonymised transcripts (1 for each focus group) were reviewed and independently coded by the first and last author using an open coding procedure. A coding frame for our NPT deductive analysis was prepared separately and data related to each construct was entered into a data coding sheet in Excel (available upon request).
Participant characteristics
Focus group interviews were conducted with 12 participants in total (7 women and 5 men; Table 1 on page 36). The first and last authors were present in all interviews and participated in conversation to varying extents; however, the findings reported here focus on patient perspectives and therefore only include quotes from the patients themselves. The median age of the participants was 69 years. Ten of the participants are now ex-smokers and 2 of the participants lifelong non-smokers. Notably, 6 of the participants have been diagnosed with COPD.
Themes
Four themes emerged from this analysis: understanding of the programme, taking part, making it happen, and possible modifications to improve implementation.
Theme 1 (coherence): understanding of the programme
The phrase ‘pulmonary rehabilitation’ had been unfamiliar to the participants prior to attending the rehabilitation programme; despite the fact, it was going to be a key aspect of their recovery:
‘I didn’t realise what I was going to be doing as such. I knew it was an exercise programme but it was really as the weeks went on we got some more information. If you knew how difficult it would have been by the end then maybe you wouldn’t have come in!’
(Participant 11, focus group 4).
A lack of awareness about the programme paired with varying attitudes towards rehabilitation led to some confusion as to whether the programme was warranted or not:
‘I really didn’t think I needed it’ (Participant 9, 3rd focus group).
Some participants shared their initial apprehension regarding feeling short of breath and some expressed the feeling that they would not have been able to partake in the PR programme for several months post resection:
‘I was nervous because I was questioning whether I would do harm to myself or if I was going to overdo it’ (Participant 10, focus group 4).

From: Exploring patient perspectives of barriers and facilitators to participating in hospital-based pulmonary rehabilitation in patients diagnosed with non-small-cell lung cancer treated with curative intent
It was very important to the participants that the importance and benefits of pulmonary rehabilitation were communicated to the participants throughout the programme:
‘They would have told us that exercise is huge and it’s such an important thing in general but that it’s particularly good for people who have had cancer and for the prevention of cancer and recurrence which really helps you want to do it a bit more.’ (Participant 11, focus group 4).
Theme 2 (cognitive participation): taking part
Participants valued an approach from staff that supported them to discover their own limitations within a safe environment and, in so doing, increased their self-confidence. It was noted that the exercise programme were individualised and tailored as needed:
‘I was going through a bad period of back and hip pain but the physiotherapist was able to amend my exercises so I can still get the
benefit from them without excess pain’ (Participant 1, focus group 1).
‘It made me realise then that I wasn’t doing enough at home and it’s okay to push yourself, that a little bit more, and If you don’t feel a little bit breathless, you’re really not doing much work. I know now I can push myself a bit more’ (Participant 11, focus group 4).
A high level of peer support was also highlighted as a main benefit of the programme within all groups:
‘I met so many people who had survived cancer with such positive attitudes and I found it really encouraging’ (Participant 3, focus group 2).
‘The social aspect is huge, and we all have something in common and we can speak to each other 1 to 1’ (Participant 2, focus group 1).
‘Oftentimes, sickness isn’t discussed which provides the mental reprieve that individuals living with cancer need.’
(Participant 2, focus group 1).
Theme 3 (collective action): making it happen
Designing and implementing a PR programme involves a wide multidisciplinary team including doctors, nurses, physiotherapists, and dieticians. These valued members of staff were available to support the programme:
‘The exercise sessions were then followed by short information sessions about nutrition, exercise, mindfulness, mental health, and respiratory disease management on alternate weeks, all of which I really enjoyed’ (Participant 1, focus group 1).
The option to have psychological support was also available:
‘I came in here to a councillor about 6 weeks after my op and they were absolutely amazing. The nurse practitioner made me aware of this service (Participant 2, focus group 1).
However, it was noted that some patients lacked the ability to partake in this programme due to other comorbid conditions:
‘I actually couldn’t do the walking as the veins in my leg are very clogged and I wasn’t a candidate for stents. What I do now is go to the pool three times a week for 40 min and I walk constantly for the 40 min. But there was a time when I couldn’t get around the supermarket.’ (Participant 5, 2nd focus group).
Some patients who were offered to join the PR programme were unable to due to the location of the hospital along with conflicting responsibilities:
‘I was living in west Clare and I’d say if I had been in Dublin, I could have gone to any classes offered to me, but of course it wasn’t practical.’ (Participant 9, 3rd focus group).
‘I wouldn’t have had the time between looking after my mother and everything else’ (Participant 4, focus group 2).
Theme 4 (reflexive monitoring): possible modifications to improve implementation
Participants agreed that partaking in the programme was valuable. The content of the talks was found to be helpful, particularly information relating to the use of inhalers and diet:
‘I found learning about inhaler technique particularly useful. There is a whole process around taking inhaled medication correctly I was unaware of. I was also educated about the use of a spacer and this helps me get the correct dose of medication. Also, it was recommended that rinsing out my mouth after taking the medication would prevent oral thrush.’ (Participant 1, focus group 1).
‘I thought the dietician was great. I’d be hearing this and that about certain foods and she explained to me there was no scientific evidence behind a lot of what I had heard but she explained the importance of fruit and veg and I have been trying to get a lot more of it in.’ (Participant 11, focus group 4).
Furthermore, patients discovered their own true limitations:
‘It made me realise then that I wasn’t doing enough at home and it’s okay to push yourself, that a little bit more, and If you don’t feel a little bit breathless, you’re really not doing much work. I know now I can push myself a bit more’ (Participant 11, focus group 4).
Discussion
The main results of this qualitative study highlighted the need to continue with relevant facilitators which included optional access to psychological support, social support, tailored programmes, and educational sessions to allow for acquisition of new knowledge. The data also highlighted that important barriers
to the effective implementation of a PR programme were patient comorbidities, location, perceived lack of benefit of PR, and a fear of causing harm. The findings of this study will be discussed in relation to the existing literature.
A significant concern noted across the literature and in this study is a lack of awareness about PR programmes and their potential benefits.11, 15 In 2014, Rochester and colleagues developed a survey to better understand patients perspectives on PR. Of 1685 respondents, 40% reported that their healthcare provider never told them about PR programmes.16 It has already been suggested in the literature that patient advocacy groups develop education materials for people living with chronic respiratory disease regarding the process and benefits of PR.10 Furthermore, the existing literature alongside this analysis confirms the need for greater healthcare professionals’ knowledge and awareness of PR to foster patient referrals.
Location of PR programmes and access difficulties are highlighted as barriers to attendance at PR programmes in this study and across the literature.15, 17 Four of twelve individuals in this analysis chose not to attend the PR programme due to conflicting responsibilities, comorbid conditions that would hamper their ability to partake, access difficulties, and feeling that the course was ‘not needed.’ Of the 8 individuals interviewed who chose to partake, there was a 100% completion rate. Travel to a PR

centre along with parking charges may act as a barrier to attendance or completion of a programme. In future, a larger number of PR centres would certainly act as a facilitator to future uptake. There is a lack of consensus across the literature about the best time to enrol in a rehabilitation programme post-treatment and no specific guidelines have been set. Rather, it has been suggested that whilst the timing of rehabilitation is crucial, it must be tailored to the individual patient and their disease process.18 Many of the participants in this study echoed the fact they felt they would have been unable for as intense a programme until many months after the surgical resection, with several of these patients partaking in the PR programme 6–12 months postsurgery. Some patients may find it distasteful to discuss rehabilitation prior to surgery when facing a lifethreatening illness. On the other hand, emphasising a proactive approach at an early time is fundamental to recovery.10
The diagnosis and treatment of lung cancer negatively affect patients in terms of the physical and emotional well-being.2 Therefore, when these patients are presented with a unique opportunity to improve their health status, they must have trust in the programmes ability to ‘do no harm.’ Not only has the safety and efficacy of PR been widely researched and supported6, 19 the strong social aspect of the course can improve mental health and establish social support networks. Those who have participated
Minister for Health Jennifer Carroll MacNeill has published the Waiting List Action Plan (WLAP) 2025, Government’s commitment to reducing waiting times for patients and improving access to hospital care.
Minister Carroll MacNeill stated, “Our people are living longer, healthier lives and will need timely and transparent access to high quality scheduled patient care in the years ahead. In the Programme for Government we committed to
further reduce waiting times which will bring a number of benefits from a patient perspective, in terms of improved outcomes and a better experience of the health service.
"The Waiting List Action Plan 2025 is an important milestone in that journey. Significant funding of ¤420 million has been allocated to the Waiting List Action Plan 2025, comprising ¤190 million for the HSE and ¤230 million for the National Treatment Purchase Fund (NTPF), which will focus on
in PR report enjoying the social aspect of training together, ultimately serving to encourage and motivate one another.20 Besides the individuals with lung cancer or chronic respiratory diseases, the caregivers might also find the desired social support in the PR setting.20
Conclusion
In this analysis, we identified that the most striking barriers to participation in PR programmes are the presence of comorbidities, location, perceived lack of benefit, and a fear of causing harm. As a result, strategies aimed at facilitating better patient education about the importance of PR alongside education of physicians on the importance of referrals to PR programmes would improve uptake rates and positive outcomes for patients and their families.
Regardless, recovery after resection of NSCLC remains a major challenge. This challenge is only exacerbated by the growing burden of obesity, diabetes, COPD, and other chronic diseases. Pulmonary rehabilitation programmes implemented by multidisciplinary teams can offer these patients a unique opportunity to improve their health status and quality of life. As we are now entering the era of personalised medicine, future large-scale studies will almost certainly be required to identify specific rehabilitation programmes most suited for specific individuals and their state of health.
Reference available on request
sustainably reducing the amount of time people are waiting for care.”
This year’s WLAP focuses on reducing waiting times for scheduled care and sets out four key targets aimed at achieving this objective, namely:
• having 50% of patients waiting less than the Sláintecare wait time targets (that is, 10 weeks for outpatients (OPD) and 12 weeks for inpatient and day case (IPDC)) by the end of 2025
• reducing the weighted average wait time for scheduled care to 5.5 months
• increasing the proportion of OPD patients waiting less than 12 months to 90% by the end of 2025
• reducing the proportion of patients waiting over 24 months, or at risk of waiting over 24 months by the end of 2025, by 90% this year
Written by Darren J. Walsh1,2, Sarah Brown3, Carina O’Brien1,4 and Ita Fitzgerald1,5
1. Pharmaceutical Care Research Group, School of Pharmacy, University College Cork, Cork
2. Pharmacy Department, University Hospital Waterford, Waterford
3. School of Science and Computing, South East Technological University, Waterford
4. Pharmacy Department, St. John’s Hospital, Limerick
5. Pharmacy Department, St. Patrick’s Mental Health Services, Dublin, Ireland

Background
In December 2024, the Hospital Pharmacists Association of Ireland (HPAI) established an Education and Research Specialist Interest Group (SIG). With the era of advanced specialism in hospital pharmacy upon us after many years of tough negotiations, we are reminded of the importance of postgraduate pharmacist education prerequisite to developing the skillsets required of advanced specialist practice. In addition to the recognition of an advanced specialist grade, the prospect of pharmacist prescribing is on the horizon. In their final report, the expert taskforce commissioned by the Department of Health to support the expansion of the role of the pharmacist recommended that “pharmacists be enabled to exercise independent, autonomous prescriptive authority within and related to the individual practitioner’s scope of practice and competence”.1 The report highlights education, training, and research as key competencies required for attaining advanced specialist status. Specific to research, the need for research conducted within Irish contexts to support regional and national implementation of taskforce recommendations was emphasised.1
Walsh
Within the report, the indicative timeline for the introduction of regulations governing pharmacist prescribing in primary and secondary care is in 2026, with pharmacist prescribing in secondary care coming into effect from 2027.
In healthcare models where pharmacists are integrated into the multidisciplinary team (MDT) and where independent pharmacist prescribing is provided for within legislation, pharmacist prescribing is widespread. Pharmacist prescribing in the hospital setting has been associated with reduced medication errors, improved prescribing safety, more efficient pharmacist medication reviews, increased scope of practice and enhanced professional or job satisfaction.1
It is hoped that the introduction of advanced specialism will also see similar opportunities to benefit patient care in Ireland via increased participation in MDT. To see such changes in practice and secondary benefits for patients, a change in workforce training is required such that pharmacists have widespread opportunities for postgraduate training and development of research skills.
The need to advance pharmacist access to education, training, and opportunities to conduct and collaborate with others in producing high quality research, provides a rationale for a SIG that incorporates both education and research into its mandate.
The European Society of Clinical Pharmacy (ESCP) extended definition of clinical pharmacy states that clinical pharmacy
“represents both a professional practice and field of research” and “encompasses cognitive, managerial and interpersonal activities targeting all stages of the medicines use process, and as a field of research generates knowledge that informs clinical decision-making, health care organisation or policy”.2
Further supporting the establishment of an education and research SIG in the HPAI are the European Association of Hospital Pharmacy (EAHP) Hospital Pharmacy Statements, which group education and research together as components of pharmacy practice that are intrinsically linked.3 Section 6: Education and Research, of the EAHP statements can be seen in Figure 1. The HPAI endorse the statements on their member forum and encourages hospital pharmacy departments to aim to achieve compliance with these statements.
Academic supervisors offer tremendous support in terms of navigating a structured MSc or PhD. However, for pharmacists conducting independent research, or whose academic supervisor’s expertise falls outside the scope of the research speciality, this network may be beneficial.
Aims and Objectives
The aims and objectives of the SIG are to enhance hospital pharmacist access to/knowledge of quality education and training opportunities and facilitate the conduct and publication of high-quality research in clinical pharmacy practice.
Education
Hospital pharmacist postgraduate course database
The SIG is looking to create a database of clinically focussed courses that are relevant to hospital pharmacists. This database will be published on the HPAI members forum. Academic institutions and HPAI members
are encouraged to share relevant postgraduate course, certificates, diplomas, and MSc programmes with the SIG, as well as any opportunities for funding streams, such as grant calls, bursaries and scholarships. Additionally, academic institutions that offer practice-based PhD programmes to hospital pharmacists are encouraged to inform the SIG to increase reach to pharmacists working in hospital settings.
Clinical competency framework for basic grade pharmacists
The SIG has identified the need for a clinical competency framework, to complement the Pharmaceutical Society of Ireland’s (PSI) core competency framework for basic grade pharmacists in their early hospital career. The PSI core competency framework is a general competency framework applicable to all pharmacists registered in Ireland. A clinical competency framework will help hospital pharmacy departments structure the career development of their early career pharmacists, as well as provide a structured roadmap for those looking to progress in their clinical practice.
Work with specialities to develop advanced specialist credentialling
A credentialling model for each area of advanced specialist pharmacy practice in the specialties acknowledged by the joint HPAI/Fórsa technical report would serve as an important quality assurance step for both pharmacists and pharmacy departments in Irish hospital pharmacy. The SIG aims to work with specialist groups of pharmacists, such as the Irish Pharmacy Haematology Oncology Society (IPHOS), to assist in the design, accreditation and rollout of this process. The specialties contained in the technical report are detailed in Table 1. Wider structural and contextual factors that have been identified as

hospital pharmacists to support development of skillsets to conduct high quality, impactful research. Examples of areas the group hopes to cover include:
• Formulating relevant and answerable research questions
facilitators or barriers to integrating advanced pharmacist credentialing into clinical practice have previously been explored by Deasy et al. The finding from this review should inform collaborative work in the future.4 These findings will be considered by the SIG as part of implementation recommendations.
Develop online and in-person workshops on conducting research in hospital pharmacy
With increasing numbers of hospital pharmacists conducting clinical research, whether structured (MSc, PhD programmes) or unstructured (e.g., independent research) the SIG intends to coordinate structured educational events for
• Developing research skills necessary for conducting primary research (i.e., developing new knowledge) and secondary research (i.e., synthesising available research through evidence synthesis).
• Specific research skills required to answer research questions, including those quantitative and qualitative methodologies
• Workshops on writing research and grant proposals
• Publishing and presenting research
Pharmacists and academics with experience in research are encouraged to join the SIG and contribute to workshop design, development, and delivery.
The SIG is keen to promote Irish hospital pharmacy research, ensuring Irish hospital pharmacists can disseminate their research widely and to appropriate audiences. This involves sharing hospital pharmacist research through the HPAI forum, social media, newsletters, pharmacy department journal clubs, and at the HPAI annual conference.
Additionally, the generation of a database of peer-reviewed publications from Irish hospital pharmacists would have benefits for researchers from a dissemination, networking, and professional culture perspective.
Peer support and mentorship for hospital pharmacy researchers
The SIG has a forum in the HPAI members website where pharmacists with research questions can be posted. In addition to this, the SIG would be interested in creating a space for peer support and mentorship for
hospital pharmacists considering starting their research journey or requiring peer support with conducting research.
Publish collaborative research relevant to Irish hospital pharmacy practice
The SIG members are planning a series of research projects in the areas of education and research within Irish hospital pharmacy practice. The first stage of this has already commenced. This entails an anonymous survey to establish research and education activities already being conducted within Irish hospitals. This will serve to benchmark current practice and allow the SIG to target members needs accordingly. Pharmacist Executive Managers are strongly encouraged to engage in survey participation.
This survey will allow SIG members to advocate for assistance in addressing gaps in education and research in collaboration with academic partners and international societies. It will also build strategic
Critical care (Intensive Care Units)
Antimicrobial stewardship
Emergency medicine/Acute medicine
Hepatitis C
Infectious diseases (including HIV)
Care of the Elderly
Paediatrics
Renal
Transplant medicine
Anticoagulation
Oncology/Haematology
Perioperative
Mental health
Maternity
Neonatal care
Neurology/stroke
Palliative care
Respiratory/Cystic fibrosis
Cardiology
Pharmacotherapy
frameworks to facilitate Irish hospital pharmacists in moving to resolve the gaps that already exist in education and research.
Aseptic compounding
Medication safety
Medicines information
Dispensary
Informatics
Education
Research
Formulary/Guideline development
Table 1: Specialties contained in the HPAI/Fórsa joint technical report
may affect hospital pharmacy technicians, and welcomes communication from other groups and bodies, in particular representatives from the clinical pharmacy departments of schools of pharmacy in different Irish universities.
The ongoing SIG activity, including publications and the provision of research and educational material will be available on the SIG forum in the HPAI members website.
References
1. Expert Taskforce to Support the Expansion of the Role of PharmacyFinal Report. Department of Health; 2024.
2. Dreischulte T, van den Bemt B, Steurbaut S, the European Society of Clinical P. European Society of Clinical Pharmacy definition of the term clinical pharmacy and its relationship to pharmaceutical care: a position paper. International Journal of Clinical Pharmacy. 2022;44(4):837-42.
part in conducting and publishing this research are encouraged to join the SIG.
Invitation for new members and collaborative groups
Wider structural and contextual factors that have been identified as facilitators or barriers to integrating advanced pharmacist credentialing into clinical practice have previously been explored by Deasy et al. The finding from this review should inform collaborative work in the future (4) These findings will be considered by the SIG as part of implementation recommendations.
Further research investigating the facilitators and barriers of structured education and research in Irish hospital pharmacy is anticipated to be the next step after completion and publication of the survey. Interested members of the HPAI who would like to take
level of experience (including none) in education and research are encouraged to join. The group currently has membership from pharmacists in the acute hospital and academic setting.
3. The European Statements of Hospital Pharmacy. European Journal of Hospital Pharmacy. 2014;21(5):256.
The SIG encourages interested parties to join by emailing the chairperson, Darren Walsh ( darren.walsh@hse.ie) to get added to the SIG mailing list. The SIG meets formally once monthly. Members of the HPAI with any
Develop online and in-person workshops on conducting research in hospital pharmacy
With increasing numbers of hospital pharmacists conducting clinical research, whether structured (MSc, PhD programmes) or unstructured (e.g., independent research). the SIG intends to coordinate structured educational events for hospital pharmacists to support development of skillsets to conduct high quality, impactful research. Examples of areas the group hopes to cover include:
• Formulating relevant and answerable research questions
The SIG has already collaborated with the National Association of Hospital Pharmacy Technicians (NAHPT) to nominate a representative who will join meetings that discuss topics that
• Developing research skills necessary for conducting primary research (i.e., developing new knowledge) and secondary research (i.e., synthesising available research through evidence synthesis).
4. Deasy E, Seoighe A, Ryan C, Byrne S, Dalton K. Pharmacy stakeholders' views and experiences of the credentialing of advanced or specialist pharmacist practice: A mixed methods systematic review. Explor Res Clin Soc Pharm. 2024;16:100522.
¤26.9bn funding allocated to reduce waiting times, improve health outcomes, improve services for people with a disability and progress in the digitisation of the health service
The Ministers for Health and Children, Equality, Disability Integration and Youth, Stephen Donnelly and Roderic O’Gorman together with Ministers of State Mary Butler, Colm Burke and Anne Rabbitte have welcomed the publication of the HSE National Service Plan 2025. The plan sets out the delivery by the HSE of a range of health and social care services that will be provided to the people of Ireland within the allocated budget of ¤26.9bn, a ¤1.6bn increase on 2024. This includes a ¤297.8m investment in new service developments including enhancing mental health, older persons and disability services.
Welcoming the publication of the National Service Plan Minister Donnelly, said, “The National Service Plan reflects the significant investment provided by government in 2025, which is the highest funding ever for the HSE. I welcome the strong focus on productivity to ensure our health service delivers as much as possible for patients within budget. I would like to thank the HSE Board and the Executive for the considerable work undertaken in delivering the National Service Plan 2025.
“It will shortly be supported by a Capital Plan involving approximately ¤1.4 billion for infrastructure and equipment across the health service. ¤190m of the Capital Plan is specifically for digital and ICT infrastructure, which will deliver on the HSE priority of advancing the Digital for Care Programme.”
According to Minister O’Gorman, “I am extremely pleased to see this year’s record investment in disability services reflected in this ambitious programme of work for the HSE. It is important that services are made more flexible and person-centered and in line with the vision of the UNCRPD. Funding provided for specialist disability services will help support this ambition. Continuing to deliver on the reform underway in disability services is key to improving the lives of the people who use those services, and this remains an important objective.
“I also welcome the range of initiatives designed to address
the known challenges within the sector and the work that the HSE will carry out, in partnership with stakeholders to strengthen recruitment and retention, make services more sustainable into the future and continue to drive change and reform across the disability sector.”
The focus of the plan includes:
• Healthy Communities: improving health and wellbeing, including through screening and protection from health threats
• Receiving the Right Care: delivery of integrated high-quality care, implementing models of care and clinical strategies
• Receiving care in the right place: providing care close to where people live and expanding alternative care pathways
• Receiving care at the right time: improving access to care, reducing wait times
• Strong foundations: expanding workforce, delivering ICT services, including the Digital for Care transformation programme. A significant programme of work that will provide patient summary data to healthcare professionals supports the administration and delivery of services/care within and across community healthcare settings and important solutions such as the single national immunisation system and the hospital medicines management system.
All of these 5 parts have specific components for both Health and Disability services. Collectively they are the framework for the HSE to deliver services and measure performance throughout 2025.
Six new Health Regions, as envisaged in Sláintecare have been established. Health and social care services will be planned and delivered around the specific needs of local populations leading to better co-ordination of care and access to services. This offers the opportunity to take the next step in creating a modern, value-based health and social care system. Finalising the change from the traditional operational centre of the HSE to a visible regional operating

system overseen by the Centre is a key target by 1 March, 2025. This will mark the completion of the most significant structural reform of the HSE and how it operates since the organisation first came into being.
Speaking about the HSE’s priorities for 2025, Ciarán Devane, HSE Chairman, said, “The Board’s overarching objectives for 2025 are to support improved efficiency and increased productivity, maintaining the ongoing focus on improving the quality of care, and continuing the shift to providing more care closer to people’s homes.
“The publication of this Plan coincides with the 20th anniversary of the establishment of the HSE. During this time Irish health and social care services have been significantly reshaped to improved health outcomes for the people of Ireland.
“While we have seen significant progress over the last 3 years, a priority for the HSE in 2025 will be to further reduce waiting times. We will place a specific focus on maximising an integrated approach to care delivery across community and acute settings, enabled through our new health regions.”
Written by Dr Orla Killeen, Consultant Paediatric Rheumatologist
Rheumatology


Juvenile Idiopathic Arthritis (JIA) refers to a group of inflammatory arthritides of unknown cause that begin before 16 years of age and persists for at least 6 weeks. It is the most common chronic paediatric rheumatological condition affecting approximately 1 in 1000 children and young people less than 16 years of age with an overall female predominance.
It is important that other causes of arthritis, including infection and malignancy, are excluded before the diagnosis is made. The key features of JIA include joint pain, stiffness, swelling and loss of movement or reduced function.
Chronic anterior uveitis is strongly associated with JIA and can occur in up to 30% depending
on the subtype of JIA. It can be asymptomatic and potentially lead to irreversible sight loss including blindness. All children with a diagnosis of JIA should be referred to ophthalmology for uveitis screening (within 6 weeks of referral) with subsequent regular uveitis monitoring by slit lamp examination. Duration of uveitis monitoring will depend on the age of the child at diagnosis and subtype of JIA, however is typically up until the age of 11-12 years (RCO/BSPAR Guidelines).
The International League Against Rheumatism (ILAR) classification criteria characterise JIA into 7 different subtypes as shown in Table 1. Oligoarticular JIA is the commonest subtype accounting

for up to 60% of all cases with the knee being the most commonly affected joint.
The heterogeneous nature of JIA adds to the complexity of fully understanding the underlying pathophysiology. It is likely a number of different risk factors, including genetic (HLA associated) and environmental (infectious triggers), are involved resulting in an auto-immune driven disease process with the production of pro-inflammatory cytokines causing synovial inflammation.
Whilst investigations can be helpful to exclude other pathologies, JIA remains a clinical diagnosis without a single diagnostic investigation. If there is clinical concern of JIA then a paediatric rheumatology referral should not be delayed even if investigations are normal.
Careful clinical assessment therefore remains essential with a detailed history and a basic musculoskeletal assessment, as a minimum, such as the paediatric Gait, Arms, Legs, Spine (pGALS). Particular focus to changes of the child’s activities, new limitations, developmental regression in addition to exploration of key features including joint pain, stiffness and swelling are important. If an
abnormality is detected on pGALS assessment, then a more detailed joint assessment should be performed such as the paediatric Regional Examination of the Musculoskeletal System (pREMS). Further information on clinical assessment and these examination methods can be found in the recommended further reading.
Elevated inflammatory markers including erythrocyte sedimentation rate, C-reactive protein and ferritin are likely to be elevated in Systemic JIA, however can be entirely normal in the other JIA subtypes. Autoantibodies can aid with prognosis and subtyping of JIA but are not diagnostic. Positive antinuclear antibodies (ANAs) in the presence of JIA can be associated with an increased risk of chronic anterior uveitis, however also occurs in other paediatric rheumatological diseases (e.g. childhood Systemic Lupus Erythematosus), nonrheumatological paediatric disorders (e.g. auto-immune hepatitis) and in healthy children.
Rheumatoid factor is rarely positive, less than 5% of all JIA patients, and more likely to be present in adolescent females with polyarticular disease predicting a more aggressive disease course and similar disease pattern to adult onset rheumatoid arthritis. Therefore, JIA should still be considered in a child presenting
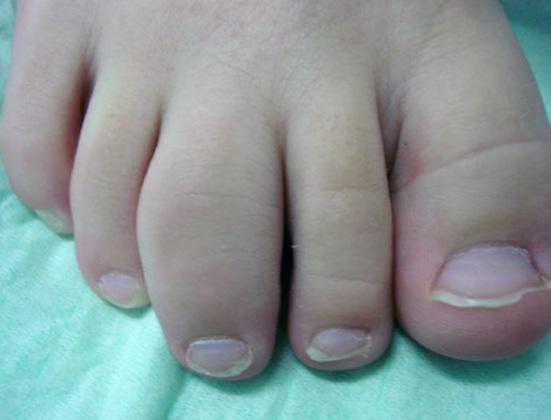
with joint symptoms with a negative rheumatoid factor. HLA B27 positivity is associated with enthesitis related JIA, however is found in approximately 20% of healthy children and can also be found in other paediatric inflammatory diseases including chronic recurrent multifocal osteomyelitis (CRMO) and inflammatory bowel disease. It is important to note that up to approximately 30% of those with enthesitis related JIA can be HLA B27 negative.
Further investigations are considered case by case and in particular to exclude alternative
Untreated JIA associated chronic anterior uveitis with band keratopathy, posterior synechiae and cataract formation
diagnoses such as infection (e.g. synovial fluid aspiration in presence of single hot swollen joint in febrile child to exclude septic arthritis) and malignancy (e.g. bone marrow aspirate in a child with joint pain and/or swelling, malaise, fever and pancytopenia).
Imaging can be helpful as part of the diagnostic work-up and later during the disease course for monitoring of disease activity
Subtype Definition
Oligoarticular JIA
Polyarticular JIA:
Rheumatoid Factor
Positive
Polyarticular JIA:
Rheumatoid Factor
Negative
Psoriatic JIA
Enthesitis Related JIA
Systemic JIA

Arthritis affecting 1 to 4 joints during the 1st 6 months of disease;
2 subcategories recognised:
1. Persistent Oligoarticular JIA: No more than 4 joints affected throughout disease course
2. Extended Oligoarticular JIA: More than 4 joints affected after the 1st 6 months of disease
Affecting 5 or more joints during the 1st 6 months. Rheumatoid factor positive on at least 2 occasions measured 3 months apart during the 1st 6 months
Affecting 5 or more joints during the 1st 6 months. Rheumatoid factor negative
Arthritis and Psoriasis or arthritis and at least 2 of:
• Dactylitis
• Nail pitting or onycholysis
• Psoriasis in 1st degree relative
Arthritis and enthesitis or arthritis or enthesitis and at least 2 of:
• HLA B27 positivity
• Sacroiliac joint tenderness and/or inflammatory lumbosacral back pain
• Onset in male over 6 years of age
• Acute symptomatic anterior uveitis
• Family history of HLA B27 associated disease
Arthritis in 1 or more joints with or preceded by fever of at least 2-week duration, documented daily for at least 3 days, accompanied by 1 or more of:
• Evanescent erythematous rash
• Generalised lymphadenopathy
• Hepatomegaly and/or splenomegaly
• Serositis
Undifferentiated JIA
Arthritis that fulfils none or in 2 of the above categories
Table 1: JIA Subtypes based on the ILAR Classification Criteria
and potential complications. X-Rays are likely to be normal initially, however can be helpful in detecting bony erosions and considering alternative causes for a child’s pain (e.g. presence of osteochondral defect).
Ultrasonography (US) can detect early or longstanding inflammatory changes within the joint and is useful in monitoring of disease activity. MSK US is well tolerated by children, can be performed rapidly at the bedside and is likely to be used in greater frequency in the next number of years.
Magnetic Resonance Imaging (MRI) ideally performed with gadolinium contrast is helpful for those where diagnostic uncertainty exists, in the presence of a normal ultrasound or monitoring for further complications. Availability can however be limited with delays in younger children requiring general anaesthetic.
Management
Principles of management include early referral to the paediatric rheumatology specialist multidisciplinary team (MDT), initiation of early treatment and monitoring for complications. Complications (such as joint damage, sight impairment, growth disturbance) are fortunately less frequently seen due to earlier diagnosis and mostly due to recent advancements in the development of biological agents to treat JIA and chronic anterior uveitis.
The MDT including physiotherapy, occupational therapy, clinical nurse specialists and psychology are all essential in the optimal management of a child with JIA. Preparing the young person with JIA for transition to adolescent services is particularly important to ensure timely transition at an
With the continued use of biological agents, the need for autologous haemopoietic stem cell transplant has reduced and is reserved for severe, refractory cases of JIA.
Generic Name Class
Etanercept TNF-α blocker
Adalimumab TNF-α blocker
Infliximab TNF-α blocker
Golimumab TNF-α blocker
Tocilizumab IL-6 blocker
Anakinra IL-1 blocker
Abatacept T-cell blocker
Route and Frequency
Subcutaneous injection once or twice weekly
Subcutaneous injection fortnightly
Intravenous infusion – initially 2 weekly and then 4-8 weekly. Also available as subcutaneous injection
Subcutaneous injection monthly
Intravenous infusion – fortnightly or monthly. Also available as subcutaneous injection
Subcutaneous injection daily
Intravenous infusion - initially 2 weekly and then monthly. Also available as subcutaneous injection
Ruxolitinib, Tofacitinib JAK inhibitor Orally twice daily
Table 2: Biological agents currently used in the treatment of JIA
appropriate timepoint for the young person with regards to their disease control and education. The diagnosis of JIA does not change and therefore should not be relabelled as rheumatoid arthritis as the patient moves to adult services.
JIA: Key Points
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are 1st line treatments for JIA. NSAIDs in isolation are unlikely to be sufficient to control the arthritis. Intra-articular corticosteroid injections (IACIs) are used for the treatment of Oligoarticular JIA and in those with polyarticular joint involvement waiting for further systemic treatment to take full effect. This lessens the potential use of high dose systemic steroids and their multiple side effects and toxicity in a growing child. In cases of severe JIA, such as systemic JIA or aggressive polyarticular JIA involving multiple joints – pulsed
intravenous methylprednisolone is likely to be needed in order to establish rapid disease control followed by a weaning course of oral prednisolone.
A separate article focusing on Systemic JIA and Macrophage Activation Syndrome will be discussed in May’s edition
• JIA is the commonest paediatric rheumatological condition. Key features include joint swelling, pain, stiffness and restricted movement
• Careful clinical assessment is necessary with consideration of alternative diagnoses such as infection and malignancy
of JIA (e.g. enthesitis related JIA) or where there is intolerance to standard DMARD therapy. Screening for infection (namely tuberculosis) before commencing and blood monitoring before and during biologic therapy is important in addition to contraception counselling and awareness of infection risk (including the avoidance of live vaccinations and recommendation of annual inactivated influenza vaccination).
• Initial investigations can often be normal and should not delay referral
Methotrexate is the most frequently used Disease Modifying Anti-Rheumatic Drug (DMARD) either in weekly subcutaneous or oral form. It is used in cases such as extended oligoarticular JIA, polyarticular JIA or psoriatic JIA where isolated IACIs are unlikely to provide sufficient control of disease.
• Chronic anterior uveitis can be asymptomatic and screening is necessary for all children with JIA
Musculoskeletal clinical assessment guide in children and young people - https:// versusarthritis.org/media/ n2wd1am4/va_paediatrichandbook-2024_digital-finalversion.pdf
References
• MDT involvement is central to the management of a child with JIA
• Intra-articular corticosteroid injections, DMARD and biologic therapy form the mainstay of treatments for JIA
• A diagnosis of JIA does not change or become relabelled as the patient moves to adolescent and adult services. Timely transition is key
JIA: Key Points
The development of biological agents to selectively inhibit the effects of pro-inflammatory cytokines have transformed the management of JIA where additional immunosuppression, to DMARD therapy, is required. Biological agents are also becoming increasingly used as 1st line treatments in some subtypes
• JIA is the commonest paediatric rheumatological condition. Key features include joint swelling, pain, stiffness and restricted movement.
• Careful clinical assessment is necessary with consideration of alternative diagnoses such as infection and malignancy.
• Initial investigations can often be normal and should not delay referral.
• Chronic anterior uveitis can be asymptomatic and screening is necessary for all children with JIA.
• MDT involvement is central to the management of a child with JIA.
• Intra-articular corticosteroid injections, DMARD and biologic therapy form the mainstay of treatments for JIA.
• A diagnosis of JIA does not change or become relabelled as the patient moves to adolescent and adult services. Timely transition is key.
Table 2 summarises the main biological agents used in the treatment of JIA. Anti-TNF therapy is typically used as a 1st line biological agent, except in systemic JIA where an antiIL-1 or IL-6 receptor antagonist is used. T cell blockage and Janus Kinase (JAK) inhibitors are reserved typically for cases that have not responded to anti-TNF therapy. Anti CD-20 therapy, such as rituximab, is less frequently used but can be considered in cases of refractory rheumatoid factor positive polyarticular JIA. Advancements in research and ongoing clinical trials are promising with the development of additional biological agents. With the continued use of biological agents, the need for autologous haemopoietic stem cell transplant has reduced and is reserved for severe, refractory cases of JIA.
A separate article focusing on Systemic JIA and Macrophage Activation Syndrome will be discussed in May’s edition
Further reading
Clinical examination including pGALS and pREMS assessment methods – Paediatric Musculoskeletal Matters (PMM) learning portfoliowww.pmmonline.org
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, MaldonadoCocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004 Feb;31(2):390-2. PMID: 14760812.
https://www.rcophth.ac.uk/wpcontent/uploads/2022/02/2006_ PROF_046_JuvenileArthritisupdated-crest-2.pdf - Accessed 20th January 2025
Zaripova, L.N., Midgley, A., Christmas, S.E. et al. Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol 19, 135 (2021). https://doi. org/10.1186/s12969-021-00629-8
McLaughlin D, Keir M, Foster H, Jandial S. Chronic arthritis in children and young people. Online Journal of Medicine. Elsevier. January 2022.
McKenna D, McLaughlin D, Campbell C, Mulholland M, Thompson A, Loughran C, Jackson P, Rooney M. Fifteenminute guide to managing oligoarticular juvenile idiopathic arthritis. Arch Dis Child Ed. 2022; 107:175-181.

The only oral once-daily calcitonin gene-related peptide (CGRP) receptor antagonist for both episodic and chronic migraine patients1,2

Tablet not actual size.
Typical characteristics of migraine are headache of unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity and association with nausea and/or photophobia and phonophobia.3
ABBREVIATED PRESCRIBING INFORMATION. AQUIPTA® (atogepant) 10 mg tablets; 60 mg tablets. Please refer to the Summary of Product Characteristics (SmPC) before prescribing. PRESENTATION: Each tablet contains: 10 mg atogepant in 10 mg tablet, 60 mg atogepant in 60 mg tablet. INDICATION: Prophylaxis of migraine in adults who have at least 4 migraine days per month. DOSAGE AND ADMINISTRATION: The recommended dose is 60 mg taken orally once daily with or without meals; swallowed whole and not split, crushed or chewed. Missed dose to be taken as soon as it isremembered. If forgotten for an entire day, missed dose to be skipped and next dose taken as scheduled. Dose Modification: The recommended dosage of atogepant with concomitant use of strong CYP3A4 inhibitors or strong OATP inhibitors is 10 mg once daily. Special Populations: Elderly: No dose adjustment needed. Renal impairment: In patients with severe renal impairment (creatinine clearance [CLcr] 15-29 mL/min), and in patients with end-stage renal disease (ESRD) (CLcr <15 mL/min), the recommended dose is 10 mg once daily. For patients with ESRD undergoing intermittent dialysis, atogepant should preferably be taken after dialysis. No dose adjustment is recommended for patients with mild or moderate renal impairment. Hepatic impairment: Avoid use of atogepant in patients with severe hepatic impairment. No dose adjustment is recommended for patients with mild or moderate hepatic impairment. Paediatric Population: The safety and efficacy of atogepant in children (< 18 years of age) have not yet been established. CONTRAINDICATIONS: Hypersensitivity to active substance or any of the excipients. SPECIAL WARNINGS AND PRECAUTIONS: Serious hypersensitivity reactions reported, including anaphylaxis, dyspnoea, rash, pruritus, urticaria and facial oedema. Most serious reactions have occurred within 24 hours of first use, however, some hypersensitivity reactions can occur days after administration. Patients should be warned about symptoms of hypersensitivity. If a reaction occurs, discontinue atogepant and institute appropriate therapy. AQUIPTA 60 mg tablets contain 31.5 mg sodium per tablet; this is equivalent to 1.6% of the WHO recommended maximum daily intake of 2 g sodium for an adult. AQUIPTA 10 mg tablets contain less than 1 mmol sodium (23 mg) per dose, that is to say essentially ‘sodium-free’. Atogepant has no or negligible influence on the ability to drive and use machines. However, if affected by somnolence, patients should exercise caution
before driving or using machinery. FERTILITY, PREGNANCY AND LACTATION: Pregnancy: Atogepant is not recommended during pregnancy and in women of childbearing potential not using contraception. Breast-feeding: It is unknown whether atogepant is excreted in human milk. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. Fertility: No human data on the effect of atogepant on fertility are available. Animal studies showed no impact on female and male fertility with atogepant treatment. UNDESIRABLE EFFECTS: Common (≥ 1/100 to < 1/10): Decreased appetite, nausea, constipation, fatigue/somnolence, weight decreased. Refer to Section 4.8 of the SmPC for details of other side effects, and for further information.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie.
LEGAL CATEGORY: POM (S1B). MARKETING AUTHORISATION NUMBERS: EU/1/23/1750/001 AQUIPTA 10 mg tablets in blisters, in cartons of 28 tablets; EU/1/23/1750/003 AQUIPTA 60 mg tablets in blisters, in cartons of 28 tablets. MARKETING AUTHORISATION HOLDER: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061, Ludwigshafen, Germany. Further information is available on request from: AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24. DATE OF REVISION: November 2024. PI/1750/002.
References: 1. AQUIPTA® Summary of Product Characteristics, available at www.medicines.ie. 2. Morena-Ajona D, et al. J Clin Med. 2022;11(6):1656. 3. Ferrari MD, et al. Nat Rev Dis Primers. 2022;8(1):2.


Prostate cancer in Europe: the statistics
Prostate cancer is the most commonly diagnosed cancer in men and the third leading cause of cancer-related deaths among males in Europe. In the European Economic Area, which includes the 26 member states of the European Union, Iceland, Liechtenstein, and Norway, with a population of 219 million men, approximately 341,000 were diagnosed with prostate cancer in 2020 (accounting for 23% of all male cancers). In the same year, around 71,000 men died from prostate cancer (representing 10% of all male cancer-related deaths). There is a significant gap between the number of new diagnoses and the number of deaths from this malignancy, which explains why about
Written by Dr Salvatore Vaccarella, Scientist - Cancer Inequalities Team
https://cancer-inequalities.iarc.who.int/
Cancer Surveillance Branch
International Agency for Research on Cancer
Figure 1. Prostate cancer incidence and mortality in Europe among men aged 35–84 years
Source: Vaccarella S et al. BMJ 2024 ; https://doi.org/10.1136/bmj2023-077738
4 million Europeans (equivalent to 1.7% of all men) are living after a prostate cancer diagnosis.
Prostate cancer screening with PSA: a controversial history
Screening is the systematic application of tests or examinations to identify individuals with a potential disease or condition within an asymptomatic population. The main goal of a population-based cancer screening program is to reduce mortality from a specific cancer or to decrease its incidence, meaning the number of new malignant cases (for example, by removing precancerous lesions, as in the case of cervical or colorectal
cancers). However, while screening aims to detect diseases early and improve outcomes, it can also lead to overdiagnosis, i.e., the diagnosis of a cancer that, if left undetected, would not have caused any negative effects on the person's health during their lifetime, nor symptoms, nor death (for example, because they grow slowly, or because the patient is affected by other diseases that will compromise their survival before the cancer itself). A screening program needs to balance benefits and harms, i.e., reduced mortality against harms, like overdiagnosis and unnecessary treatments.
In the case of prostate cancer, screening with the PSA test (prostate-specific antigen) aims to reduce mortality from prostate cancer, but it can lead to overdiagnosis and overtreatment. Studies have shown that up to one-third of men of screening age might have prostate cancer that will never produce symptoms. The two largest randomized studies on prostate cancer screening with PSA have shown conflicting results, and due to the delicate balance between risks and benefits demonstrated by these studies, almost all European countries, except for Lithuania, have chosen not to implement population-based screening programs for prostate cancer.
Instead, they have preferred to promote shared decision-making between healthy adults (i.e., without symptoms attributable to prostate cancer) and their doctors regarding the frequency and age at which to conduct PSA tests.
Individual attitudes and local practices regarding the PSA test, in a context of opportunistic screening without clear protocols, may lead to a different balance between benefits and risks at the population level than what was observed in randomized clinical trials. In particular, there is concern that this approach may result in fewer benefits (in terms of lives saved) and more risks of overdiagnosis.
A recent IARC population-based study
Recently, we at the International Agency for Research on Cancer (IARC), in collaboration with researchers from institutions including the Centro di Riferimento Oncologico di Aviano and Sun Yat-sen University in Guangzhou, China, have analysed the epidemiological patterns of prostate cancer incidence and mortality across 26 European countries. We have assessed how incidence rates vary geographically and over time, also considering the frequency of PSA testing in each population.
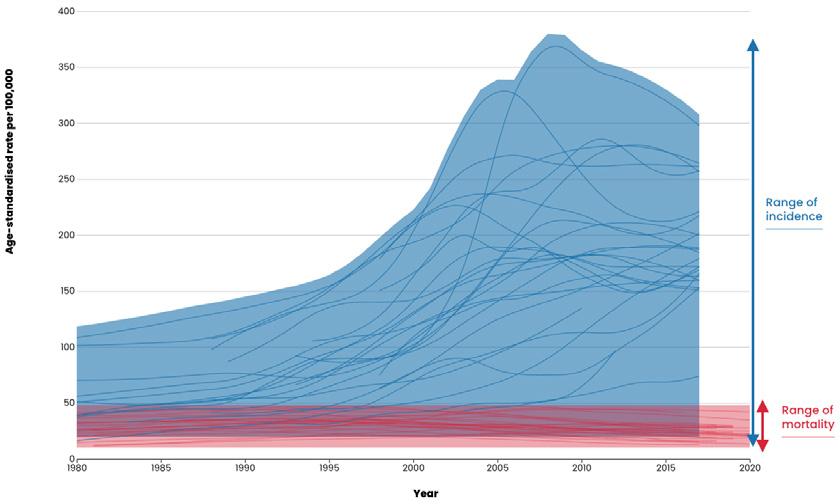

Between 1980 and 2017, prostate cancer incidence rates increased across Europe, but the magnitude of this rise varied significantly between countries (Figure 1). In contrast, mortality rates were much lower and exhibited far less variation compared to incidence rates. Most countries experienced a consistent decline in mortality, with smaller temporal differences among nations. During the study period, a twenty-fold difference in incidence rates was observed between the countries with the highest and lowest incidence, while the variation in mortality rates between countries was fivefold (Figure 1).
Temporal correlation between prostate cancer incidence and PSA testing use
It is particularly significant that, in each country, the variation in prostate cancer incidence rates closely followed the temporal changes in the frequency of PSA testing, as shown in Figure 2.
Figure 2. Temporal variation in prostate cancer incidence rates in relation to the temporal trends of PSA testing. Source: Vaccarella S et al. BMJ 2024 ; https://doi. org/10.1136/bmj-2023-077738
Interpretation of results
These findings, based on consistent population data, are consistent with a significant overdiagnosis of prostate cancer, driven by opportunistic screening with the PSA test, and suggest a possible minimal reduction in mortality.
This study is particularly relevant given that the European Union, through the "Europe’s Beating Cancer Plan" has recently issued recommendations for a new prostate cancer screening strategy. Specifically, the EU suggests that countries proceed gradually, starting with pilot projects and conducting further research to understand whether it is possible to organize programs that effectively contain overdiagnosis. These programs propose PSA testing (a blood test) for men up to 70 years old, along with MRI scans as a follow-up test in cases of elevated PSA values. The use of MRI before biopsy and targeted prostate biopsies, as opposed to systematic biopsies, is expected to reduce the risk of overdiagnosis or unnecessary treatments for diseases that are indolent and do not require intervention.
Figure 2. Temporal variation in prostate cancer incidence rates in relation to the temporal trends of PSA testing.
Source: Vaccarella S et al. BMJ 2024; https://doi.org/10.1136/bmj-2023-077738
Given the already high levels of overdiagnosis, and the uncertainty about how MRI usage can reduce it, the national healthcare system, supported by the medical-scientific community, will need to implement these recommendations carefully. This should include considering the impact on waiting lists and ensuring, as much as possible, that individuals do not seek these tests privately, which would exacerbate disparities between those who can afford healthcare costs and those who cannot.
The results of this new study suggest that spontaneous screening programs for prostate cancer offer limited benefits in terms of population-level mortality reduction but result in a high rate of overdiagnosis among men. The potential implementation of national or regional organized prostate cancer screening programs must be approached with great caution to minimize the harms of overdiagnosis, which is already widespread in Europe.
Even if the use of MRI reduces overdiagnosis, it is still unclear to what extent this can be mitigated, making it essential to carefully evaluate the risks and benefits of such programs and continuously monitor them through population studies and cancer registries.
Vaccarella S, et al. Prostate cancer incidence and mortality in Europe: a baseline for proposed prostate cancer screening programmes in the European Union. BMJ, 4 September 2024 ; https://doi. org/10.1136/bmj-2023-077738


NCI Cancer Consortium (AICC) grant. This project, led by Dr Kathy Gately, a senior clinical lecturer at the Trinity St. James
island of Ireland in collaboration with National Cancer Institute (NCI) experts at Johns Hopkins University (JHU), Baltimore, US.


Written by Dr Kathy Gately
The project was co-created in consultation with clinician scientists (Prof Patrick Forde (TSJCI/JHU) and Prof Valsamo Anagnostou (JHU)) and an AllIreland team of thoracic surgeons (Mr Gerard Fitzmaurice and Mr Rory Beattie), pathologists (Prof Stephen Finn & Prof John O’Leary), clinicians (Prof Jarushka Naidoo & Dr Aidan O’Brien), translational research scientists (Prof Paul Mullan, Prof Lorraine O’Driscoll, Dr Ezgi Oner & Dr Sharon O’Toole), research nurse (Ms Sinead Hurley), patient advocate Mr Seamus Cotter, bioinformatician (Dr Pilib O’Broin) and the Northern Ireland Biobank. Each providing firsthand knowledge of the needs of the Irish health research and services system North and South. The project will hire a research




assistant and post-doctoral fellow who will initially focus on advancing liquid biopsy research for patients with lung cancer with the goal of expanding to other cancer types.
The liquid biopsy (LB) has emerged as an important minimally invasive tool to detect micrometastatic disease and assess changes longitudinally at more frequent intervals. The LB tumor circulome comprises circulating tumor cells, tumor DNA (mutated/methylated), RNA, proteins and extracellular vesicles (EVs) that are shed from the tumor and are implicated in various cancer processes. Cell-free DNA (cfDNA) released into the blood due to apoptosis, necrosis and phagocytosis is known as circulating tumor DNA (ctDNA) when it originates from tumor cells. ctDNA is the most extensively studied component of the LB and is used to identify patients harboring minimal residual disease (MRD) following curative treatment and guide the administration of adjuvant therapy. Our goal is to co-analyse the multiple LB components in longitudinal patient samples.
The TransAtlantic Cancer Alliance will expand and add value to the HEA funded, All-Ireland
Figure 1.0 The TransAtlantic Cancer Alliance










Liquid Biopsies Consortium (CLuB) (Figure 1). Together, novel circulating tumor biomarkers and predictive signatures identified will be developed as diagnostic assays to support personalised medicine approaches for patient care. The Alliance will also establish an interorganisational scientific exchange programme for innovative training and education opportunities, for the next generation of LB leaders.
The post-doctoral fellow working on this project and two additional researchers, will have the opportunity to undertake LB research and training, in Prof Anagnostou’s molecular oncology laboratory, at the Sidney Kimmel Cancer Center at JHU.
The postdoc will train for 2 years and the two researchers for 2 months at JHU and will facilitate knowledge transfer upon their
return to Ireland. Anagnostou leads a dynamic and diverse group focusing on studying tumor evolution under selective pressure of cancer therapies, especially immunotherapy. She has discovered novel genomic mechanisms of response and resistance to cancer immunotherapy and tied those to evolutionary trajectories of ctDNA in blood.
Her team are particularly interested in translating scientific discoveries in clinical cancer care, with clinical trials underway that capitalize on their liquid biopsy discoveries.
Ongoing studies include genomic mechanisms of response and resistance to immunotherapy, cancer cell and tumor microenvironment evolution, development of minimally invasive methodologies to capture MRD

and identification of tissue and blood-derived genomic biomarkers of response to therapies.
Anagnostou has a keen focus on mentorship and career development of lab members and employs a personalised mentorship approach. The postdoc will have the opportunity to participate in the International Society for Liquid Biopsy (ISLB) annual meetings, present their work, and engage with world renown experts as well as participate in the ISLB Young Committee, which offers additional mentorship, networking and professional development opportunities, aimed at empowering the post-doctoral fellows to become future leaders in the field. Secondments to JHU will be an integral part of the TransAtlantic Cancer Alliance’s
The Institute of Medicine Spring Symposium, held on 23 January at No. 6 Kildare Street, focused on coaching and communication in medical education. With engaging talks from experts, the symposium highlighted the importance of coaching and the nuances of virtual communication.
Dr Maya Hammoud, Professor of Obstetrics and Gynecology and Learning Health Sciences, University of Michigan Medical School and Senior Advisor, American Medical Association, delivered an insightful talk on coaching in medical education. She defined coaching and differentiated it from advising, mentoring, and other traditional faculty roles, emphasising that coaching is not about giving direct answers but about helping individuals uncover their own goals and pathways. Dr Hammoud stressed the importance of coaching with compassion rather than coaching for compliance, highlighting a fundamental shift in medical training. She outlined a strength-based coaching approach.
The symposium also tackled the challenges of virtual communication, with Ms Claire Doole, Communications Trainer and Conference Moderator, sharing her expertise on engaging
Dr Emer Kelly, Institute of Medicine Director of Education and Training with Dr Maya Hammoud, Professor of Obstetrics and Gynaecology and Learning Health Sciences, University of Michigan Medical School and Senior Advisor, American Medical Association
audiences effectively over Zoom. Ms Doole highlighted common difficulties in virtual presentations such as passive audiences, lack of non-verbal cues, technical issues, and time management difficulties. She emphasised the importance of establishing an effective virtual presence by enhancing three key channels of communication: visual, vocal and verbal. Ms Doole also outlined key principles for making messages stick: keeping them simple, unexpected, credible, concrete, emotional, and story-driven.
The symposium concluded with updates from the Institute. Prof Edward McKone spoke about the Irish Clinician Educator Training (ICET) Programme, followed by reflections on training from Dr Jeffrey Harte, 4th-year Specialist Registrar in Infectious Diseases and GIM and Dr Karen Dennehy, 4thyear Geriatric Specialist Registrar.
education and training programme and will provide a unique opportunity for significant career development for researchers.
This specialised training, generously supported by Prof Anagnostou and JHU, will establish an impactful mechanism for inter-organisational, knowledge exchange between research leaders at the Sidney Kimmel Cancer Center and All-Ireland Cancer Centres and academic partners.
This unique collaboration brings together leading experts with a wealth of diverse knowledge and experience to further strengthen and sustain the development of a transatlantic, cross-border, network for clinical cancer research and innovation to advance the LB in the clinic for personalised patient care.
The ICET programme is an innovative, advanced, educational pathway in the field of Postgraduate Clinical Education. It is a two-year National Programme developed to provide training in Postgraduate Clinical Education and it is open to Specialist Registrars who can undertake it as Out of Clinical Programme Experience (OCPE) in alignment with RCPI regulation for OCPE.
Professor Edward McKone, Institute of Medicine Foundation Dean



The All-Ireland Liquid Biopsy Consortium (CLuB; www.clubcancer.ie) is a collaborative program focused on advancing liquid biopsy technologies for cancer diagnosis and treatment. It unites researchers, clinicians, patients and institutions across both Northern Ireland and the Republic of Ireland. The consortium aims to develop minimally invasive, costeffective, and reliable liquid biopsy methods that could complement or, in some cases, replace traditional tissue biopsies. These blood tests seek to enable earlier and more precise diagnoses, support personalised treatment strategies, and enhance patient outcomes. By combining expertise across the island, the consortium accelerates innovation in the field of liquid biopsy.
CLuB is led by Prof Lorraine O’Driscoll in Trinity College Dublin and the Northern Ireland lead is Prof Paul Mullan in Queens University Belfast (QUB). It is funded by the Higher Education Authority under the North South Research Programme, emerging
Written by Sharon O’Toole, Senior Research Fellow, Departments of Obstetrics and Gynaecology/ Histopathology, Trinity Centre, St. James's Hospital
hub of excellence. The premise of CLuB was to amalgamate the fragmented research that was happening across the country so that the information is maximised for liquid biopsies from patients. This information would then be processed by data scientists in the University of Galway under the direction of Dr Pilib Ó Broin to develop better algorithms for diagnosis and treatment.
CLuB focuses on a number of components of the liquid biopsy, namely;
• Circulating tumours cells (CTCs) – cancer cells that have detached from a primary tumor and are circulating in the blood. (Dr Sharon O’Toole, Prof John O’Leary and Dr James Beirne lead the ovarian strand and Dr Kathy Gately leads the lung strand)
• Circulating tumour DNA (ctDNA) – fragments of DNA shed from tumors into the bloodstream. (Prof Paul Mullan)
• Extracellular vesicles – small vesicles released from cells, including tumour cells, that carry various molecular signals. (Prof Lorraine O’Driscoll)
• Other biomarkers – such as proteins that may indicate the presence of cancer or provide an insight into the tumour behaviour.
In addition to blood samples, CLuB under the direction of Dr Niamh Buckley in QUB, also works with excised tumor tissue/organoids as "avatars" to better understand the origin of components present in liquid biopsies and test the effectiveness of potential treatments.
Ovarian cancer is one of a number of cancers being investigated within the CLuB consortium which also includes lung, breast, pancreatic and gastric and has the ambition to expand to other cancer types in the interest of all patients with cancer.
Ovarian cancer is one of five gynaecological cancers (vulva, vaginal, cervix, uterine and ovarian) but it has the highest mortality of the gynaecological cancers due to late presentation of disease and the development of chemoresistance. Annually, approximately 400 women are diagnosed with ovarian cancer, and almost 300 women die from this disease in the Republic of Ireland (National Cancer Registry Ireland). In Northern Ireland, almost 300 are diagnosed and 128 women die (Northern Ireland Cancer Registry). Ovarian cancer is the fourth leading cause of cancer death in women in Ireland, after lung, breast, and colorectal cancer. Currently there is no screening for ovarian cancer and current diagnostics lack sensitivity and specificity. Symptoms of ovarian cancer can be vague and CLuB realises the importance of awareness initiatives and supports the work of the Irish Network for Gynaecological Oncology (https://isgo.ie/irishnetwork-for-gynaecological-
oncology/) in their award winning BEAT awareness campaign.
• Bloating that is persistent and doesn’t come and go
• Eating less and feeling full more quickly
• Abdominal and pelvic pain you feel most days
• Toilet changes in urination or bowel habits
CLuB researchers Dr Brian Henderson and Ms Faye Lewis working alongside Dr Mark Ward are focused on novel diagnostics for ovarian cancer but also looking at the role of the liquid biopsy for helping guide the treatment for ovarian cancer patients to enable a more precision medicine-based approach. CLuB is following patients longitudinally throughout their treatment, with the help of our research nurse Sinead Hurley and researcher Marika Kanjuga, to determine the role of the biomarkers in predicting response to treatment.
Embedded in CLuB is the patient voice and CLuB are fortunate to have the input of a passionate and dynamic patient advocacy panel who are excited at the prospect of developing novel liquid biopsy tests to improve patient outcomes (Jacqueline Daly, Krista Costello, Roberta Horgan and Gemma Ward, coordinated by CLuB project manager Dominique Plant).
CLuB is grateful to our funders (Higher Education Authority North South Research Programme), the clinicians who consent and to all the patients who donate their samples for research.



Zejula is a PARP inhibitor approved for women with advanced ovarian cancer in response to first-line platinum-based chemotherapy, regardless of biomarker status*1—2
ZEJULA is indicated as monotherapy for the maintenance treatment of adult patients with advanced epithelial (FIGO stages III and IV) high-grade ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy.1 Overall population: median PFS of 13.8 months for ZEJULA vs 8.2 months for placebo (HR: 0.62 [95% CI: 0.50–0.76], P<0.001).
PRIMA was a randomised, double-blind, placebo-controlled Phase III trial examining the efficacy and safety of ZEJULA in patients who responded to first-line platinum-based chemotherapy.1,3
Zejula 100 mg film-coated tablets Abbreviated Prescribing Information (Refer to Summary of Product Characteristics (SmPC) before prescribing).
PRESENTATONS: Each tablet contains niraparib tosylate monohydrate equivalent to 100 mg of niraparib. Each tablet contains 34.7 mg lactose. INDICATIONS: 1. Monotherapy for the maintenance treatment of adult patients with advanced epithelial (FIGO Stages III and IV) high-grade ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy. 2. Monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed high grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. POSOLOGY AND ADMINISTRATION: Treatment should be initiated and supervised by a physician experienced in the use of anticancer medicinal products. Zejula tablets should be taken without food (at least 1 hour before or 2 hours after a meal) or with a light meal. Patients should be encouraged to take their dose at approximately the same time each day. Bedtime administration may be a potential method for managing nausea. First-line ovarian cancer maintenance treatment: 200 mg taken once daily. However, for those patients who weigh ≥ 77 kg and have baseline platelet count ≥ 150,000/μL, the recommended starting dose is 300 mg, taken once daily. Recurrent ovarian cancer maintenance treatment: 300 mg once daily. If patient weighs less than 58 kg a starting dose of 200 mg may be considered. Treatment should be continued until disease progression or toxicity. Missing dose: If patients miss a dose, they should take their next dose at its regularly scheduled time. For dose modifications due to adverse reactions see SmPC. Elderly (≥ 65 years): No dose adjustment necessary; limited clinical data in patients aged >75 years. Renal impairment: No dose adjustment with mild to moderate renal impairment. Use with caution in severe renal impairment or end stage renal disease undergoing haemodialysis. Hepatic impairment: No dose adjustment with mild hepatic impairment (either aspartate aminotransferase (AST) > upper limit of normal (ULN) and total bilirubin (TB) ≤ ULN or any AST and TB > 1.0 x – 1,5 x ULN). For patients with moderate hepatic impairment (any AST and TB > 1.5 x - 3 x ULN) the recommended starting dose of Zejula is 200 mg once daily. Use with caution in patients with severe hepatic impairment (any AST and TB > 3 x ULN) patients. Patients with ECOG performance status 2 to 4: No clinical data. Paediatric population: No data available. CONTRAINDICATIONS: Hypersensitivity to niraparib or to any of the excipients. Breast-feeding. WARNINGS/PRECAUTIONS: Haematologic adverse reactions: Complete blood counts should be done weekly for the first month, followed by monthly monitoring for the next 10 months and periodically after this time is recommended to monitor for clinically significant changes in any haematologic parameter. If a severe persistent haematologic toxicity develops that does not resolve within 28 days following interruption, Zejula should be discontinued. Myelodysplastic syndrome/acute myeloid leukaemia (MDS/AML): For suspected MDS/AML or prolonged haematological toxicities the patient should be referred to a haematologist. If MDS/AML is confirmed, Zejula should be discontinued, and the patient treated appropriately. Hypertension, including hypertensive crisis: Pre-existing hypertension should be adequately controlled before starting Zejula treatment. Blood pressure should be monitored at least weekly for two months and monitored monthly afterwards for the first year and peri-
odically thereafter during treatment with Zejula. Hypertension should be medically managed as well as adjustment of the Zejula dose if necessary. Zejula should be discontinued in case of hypertensive crisis or if medically significant hypertension cannot be adequately controlled. Posterior Reversible Encephalopathy Syndrome (PRES): PRES is a rare, reversible, neurological disorder which can present with rapidly evolving symptoms including seizures, headache, altered mental status, visual disturbance, or cortical blindness, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably magnetic resonance imaging (MRI). In case of PRES, it is recommended to discontinue Zejula and to treat specific symptoms including hypertension. The safety of reinitiating Zejula therapy in patients previously experiencing PRES is not known. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take Zejula. Patients who take Zejula may experience asthenia, fatigue, dizziness or difficulties concentrating. Patients who experience these symptoms should observe caution when driving or using machines. INTERACTIONS: Caution in combination with vaccines, immunosuppressant agents or with other cytotoxic medicinal products. Caution when combined with active substances the metabolism of which is CYP3A4-dependent and, notably, those having a narrow therapeutic range (e.g. ciclosporin, tacrolimus, alfentanil, ergotamine, pimozide, quetiapine, and halofantrine). Caution when combined with active substances the metabolism of which is CYP1A2-dependent and, notably, those having a narrow therapeutic range (e.g. clozapine, theophylline, and ropinirole). Caution when combined with substrates of BCRP (irinotecan, rosuvastatin, simvastatin, atorvastatin, and methotrexate). Increased plasma concentrations of co-administered medicinal products that are substrates of MATE1 and -2 (e.g. metformin) cannot be excluded. Caution when niraparib is combined with active substances that undergo an uptake transport by OCT1 such as metformin. Fertility, pregnancy and lactation: Fertility: No clinical data. Zejula should not be used during pregnancy or in women of childbearing potential not willing to use highly effective contraception during therapy and for 6 month after receiving the last dose. A pregnancy test should be performed on all women of childbearing potential prior to treatment. Pregnancy: Zejula should not be used during pregnancy. Breast-feeding: Contraindicated during administration and for one month after last dose. UNDESIRABLE EFFECTS: Very common (≥ 1/10): UTI, thrombocytopenia, anaemia, neutropenia, leukopenia, decreased appetite, insomnia, headache, dizziness, palpitations, hypertension, dyspnoea, cough, nasopharyngitis, nausea, constipation, vomiting, abdominal pain, diarrhoea, dyspepsia, back pain, arthralgia, fatigue, asthenia. Common (≥ 1/100, < 1/10): Bronchitis, conjunctivitis, MDS/AML, hypersensitivity, hypokalemia, anxiety, depression, cognitive impairment, dysgeusia, tachycardia, epistaxis, dry mouth, abdominal distension, mucosal inflammation, stomatitis, myalgia, photosensitivity, rash, peripheral oedema, increased GGT, AST, ALT, AKK Phos, creatinine, decreased weight. For more details on undesirable effects, see SmPC. Marketing Authorisation (MA) Holder: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nr: EU/1/17/1235/004. Legal category: POM A. Date of preparation of API: January 2024. Code: PI-12578. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie . Adverse events should also be reported to GlaxoSmithKline on 1800 244 255
Abbreviations: CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; HRd, homologous recombination deficient; PARP, poly(ADPribose) polymerase; PFS, progression-free survival.
References: 1. Zejula (niraparib). Summary of Product Characteristics, December 2022 https://www.medicines.ie/medicines/zejula-100-mg-hard-capsules-35060/spc last accessed: April 2023. 2. Olaparib. Summary of Product Characteristics. 3. González-Martín A, Pothuri B, Vergote I, et al.; for the PRIMA/ENGOT-OV26 GOG-3012 Investigators. N Engl J Med. 2019;381(25):2391—2402.
Trade marks are owned by or licensed to the GSK group of companies ©2024 GSK group of companies or its licensor. PM-IE-NRP-JRNA-230004 December 2024

Written by Éanna J. Ryan1, Ronan A. Cahill1,2
1Department of Colorectal Surgery, Mater Misericordiae University Hospital, Dublin, Ireland
2UCD Centre for Precision Surgery, School of Medicine, University College Dublin, Dublin, Ireland
Corresponding author: Professor Ronan Cahill - Full Professor of Surgery University College Dublin, Consultant Colorectal Surgeon Mater Misericordiae University Hospital. ronan.cahill@ucd.ie


Ronan A. Cahill
Introduction
The management of rectal cancer in Ireland has evolved significantly in recent years, driven by advancements in imaging, therapeutic modalities including surgical techniques and a growing focus on personalised care. While surgery remains the primary treatment for rectal cancer confined to the bowel wall, for the past two decades, neoadjuvant long-course chemoradiotherapy (CRT) has become the cornerstone of treatment for locally advanced rectal cancer (LARC), aiming to downstage tumours to improve resectability and reduce recurrence rates.1-5
The potential for organ-preserving approaches in complete or near complete clinical responders (cCR and CR respectively) resulting from this adjuvant treatment has been increasingly utilised, sparing selected patients the morbidity of radical surgery, meaning that there has been increased consideration of “neoadjuvant therapy” as “primary therapy”. Most recently the concept and practice of Total Neoadjuvant Therapy (TNT), has opened new opportunities to integrate systemic chemotherapy earlier in the treatment pathway. This approach has demonstrated improved outcomes for patients
with high-risk disease, including better systemic control and increased rates of pathological complete response (ypCR).6
There is increasing interest in such treatment with the specific aim of achieving cCR and avoiding surgery altogether.7, 8 Simultaneously, technological advancements in surgical approaches, particularly the incorporation of robotic-assisted and transanal surgical techniques have expanded the surgical armamentarium for rectal cancer resection meaning both extended and localised procedures are enabled as either definitive or significant component of cancer care.9, 10
Recently the NCCP addressed the growing complexity of the multimodality treatment by producing an updated guideline (2024 update to National Clinical Guidelines No 25: Diagnosis, Staging and Treatment of Rectal Cancer) providing evidence based recommendations on the neoadjuvant treatment of patients with LARC. This update supersedes the 2020 recommendations and reflects advancements in clinical practice and emerging evidence including those regarding TNT and neoadjuvant chemotherapy (nCT) but given the acceleration
in the field perhaps especially in oncology a further update in a shorter interval is planned.
Summary of the NCCP 2024 update to the NCCP Clinical Guideline No. 25 for the diagnosis, staging and treatment of rectal cancer:
Objectives of the Updated Guideline
The updated guideline aims to:
1. Incorporate Recent Evidence: Synthesise findings from highquality randomised controlled trials and international guidelines.
2. Standardise Clinical Practice: Reduce variability in neoadjuvant treatment strategies for LARC across Ireland.
3. Emphasise Patient-Centered Care: Integrate patient preferences and individual treatment goals into decision-making.
4. Improve Oncologic Outcomes: Enhance overall survival (OS), disease-free survival (DFS), and pathological complete response (ypCR) rates while minimising morbidity.
Methodology of the Guideline Update
The updated guideline for the neoadjuvant treatment of LARC was developed through a systematic process to ensure its clinical validity and applicability. A multidisciplinary team of experts, including colorectal surgeons, oncologists, radiologists, pathologists, nurses, and patient advocates, formed the Guideline Development Group (GDG). The guideline focused on addressing key clinical questions, structured using the Population, Intervention, Comparison and Outcome (PICO) framework. The primary aim was to identify patient subgroups benefiting from neoadjuvant therapies and evaluate the efficacy of TNT and nCT compared to standard approaches like nCRT.
Evidence Review and Appraisal
A systematic literature search identified relevant studies, including randomised controlled trials (RCTs) and meta-analyses published between 2015–2023. The evidence was appraised for quality using validated tools like the SIGN checklist, focusing on internal validity, statistical significance, and clinical relevance. Ten major trials including RAPIDO, OPRA, PRODIGE-23, CA0/AR)/AIO12 and PROSPECT, along with international guidelines, were included. Recommendations were developed using the GRADE framework, assessing evidence quality, benefits versus harms, patient preferences, and resource implications. Recommendations were classified as strong or weak based on the balance of effects, with additional good practice points provided for practical considerations.
Stakeholder Consultation, Approval and Implementation
The draft guideline underwent national and international review. Feedback was collected from professional organisations, academic institutions, and external reviewers with expertise in rectal cancer management. Revisions were made based on consensus. The final draft was quality-assured by the National Cancer Control Programme (NCCP) and ratified by its executive committee. Dissemination strategies included distribution through professional networks, the HSE website, and education programs for multidisciplinary teams. A plain language summary was also created for patients.
Monitoring and Review
Implementation is planned to be supported by regular audits and monitoring of clinical outcomes. The guideline is scheduled for review in three years, with interim updates as new evidence emerges.
Patient Eligibility Criteria
Patients eligible for neoadjuvant therapy were defined as those with locally advanced, operable rectal cancer, with eligibility determined by a multidisciplinary team (MDT) based on the following criteria:
1. Tumour Characteristics
risk of circumferential resection margin (CRM) involvement.
2. Radiologic Findings
• Baseline Imaging:

o CT: Identification of distant metastases.
3. Clinico-Pathological Risk Factors
• CRM positivity or proximity.
• Comorbidities and Age: Consideration of overall functional status and tolerability of therapy.
5. Patient Preferences
• Oncologic control with curative intent, optimizing tumour resectability and minimizing recurrence risks.
• Staging: Tumours classified as cT2 N+ M0, cT3-4 N any M0, or other high-risk categories based on TNM classification.
o MRI: Assessment of mesorectal fascia (MRF) involvement, extramural vascular invasion (EMVI), and tumour proximity to the anal verge.
• EMVI or enlarged lateral lymph nodes.
• Tumour fixation or involvement of adjacent structures.
4. Patient Factors
• Desire for organ preservation and tolerance for treatmentrelated toxicities.
6. Treatment Goals
• Location: Tumours involving the distal, middle, or upper third of the rectum, particularly those at
o Endorectal Ultrasound (ERUS): Detailed evaluation of tumour depth and nodal involvement.
• Performance Status: ECOG/ WHO scores of 0–2 in most clinical trials.
Table 1: Summary of Clinical Trials in Neoadjuvant Treatment
Summary of Patient Eligibility Criteria in Trials
Summary of Patient Eligibility Criteria in Trials
(13) n=599 cT34 Any M0 Distal or middle third
RAPIDO(14,15) n=912 T4a Any M0 <16cm from anal verge
Any N2 M0 <16cm from anal verge
PRODIGE 23 (16) n=461 cT34 N Any M0 <15cm from anal verge
CAO/ARO/ AIO-12( 17) n=311 cT4 Any M0 ≤12cm from anal verge
cT N+ M0 ≤12cm from anal verge
KCSG CO 14-03 (18) n=110 cT34 Any M0 ≤12cm from anal verge
POLISH II (19) n=541 cT34 Any M0 ≤12cm from anal verge
GCR-3 (20) n=108 cT34 Any M0 ≤12cm from anal verge
• Oncologic control with curative intent, optimising tumour resectability and minimising recurrence risks.

The guideline identified TNT and nCT as primary modalities for managing LARC, emphasising their potential to improve oncologic outcomes and treatment adherence.
1. TNT
TNT integrates both systemic chemotherapy and radiotherapy in the preoperative setting, differing from traditional approaches where systemic therapy is administered postoperatively. TNT can be delivered as:
• Induction: Chemotherapy followed by long-course chemoradiotherapy (LCRT).
• Consolidation: LCRT followed by systemic chemotherapy.
Consolidation Total Neoadjuvant Therapy (TNT)
• STELLAR, RAPIDO, POLISH II: Comparable or better DFS with TNT compared to chemoradiotherapy. RAPIDO demonstrated reduced diseaserelated treatment failures.
• OS Improvement: Observed in STELLAR and POLISH II but not in RAPIDO.
• ypCR Rates: Significantly improved in STELLAR and RAPIDO; trends favoring TNT in POLISH II and KCSG CO 14-03.
Induction Total Neoadjuvant Therapy (TNT)
• PRODIGE-23 (Conroy et al., 2021): Significant improvement in DFS and higher ypCR rates with TNT; trend towards improved OS (not statistically significant).
• GCR-3 (Fernandez-Martos et al., 2015): No significant difference in DFS or OS between TNT and chemoradiotherapy.
2. Sequencing Strategies:
• OPRA: Consolidation chemotherapy following radiotherapy achieved better TME-free survival compared to induction chemotherapy.
• CAO/ARO/AIO-12: Supported consolidation chemotherapy, reporting higher ypCR rates and DFS improvements.
3. Neoadjuvant Chemotherapy (nCT)
nCT involves the administration of systemic chemotherapy without radiotherapy prior to surgery. It offers a potential alternative for patients who may not require radiation therapy, thereby reducing associated toxicities.
Evidence Supporting nCT
• Key Trials:
o CONVERT (Mei et al., 2023): Similar ypCR rates between neoadjuvant CAPOX chemotherapy and chemoradiotherapy.
o PROSPECT (Schrag et al., 2023): Chemotherapy is noninferior to chemoradiotherapy for DFS and OS.
Patient Selection for nCT
• Appropriate for patients with lower-risk disease and minimal risk of CRM involvement.
• Radiotherapy can be introduced later if an adequate response to chemotherapy is not achieved.
Recommendations for Clinical Practice
1. Multidisciplinary Team (MDT) Approach
o Treatment decisions should be made during MDT conferences, integrating radiologic, pathologic, and clinical data.
o MDT recommendations should address:
• Tumour location and staging.
• Imaging findings, including CRM involvement and EMVI.
• Patient-specific factors, such as comorbidities, age, and treatment preferences.
2. Individualised Treatment
o Shared decision-making is essential, ensuring detailed discussions on the risks, benefits, and treatment objectives for each patient.
o Non-operative management (e.g., watch-and-wait (W&W) strategy) should be considered for patients demonstrating a cCR following TNT.
Practical Considerations for Implementation
1. Patient Education
o Clear and continuous communication about treatment options, expected outcomes, and potential side effects is vital.
o Provision of a plain language summary can enhance patient understanding and engagement.
2. Multidisciplinary Communication
o Active participation of multiple MDT members during
consultations allows patients to address their questions at different stages of care.
3. Resource Allocation
o The need for increased radiological and endoscopic follow-up for patients opting for a W&W approach is anticipated.
o Sufficient consultation time and access to specialised oncology services to support patient-cantered care needs to be ensured.
4. Audit and Monitoring
o Cancer centres should conduct regular audits to assess adherence to the guidelines and evaluate patient outcomes.
Benefits and Harms
Benefits
1. Improved Complete Response Rates: Total neoadjuvant therapy (TNT) or neoadjuvant chemotherapy (nCT) may enhance the likelihood of tumour shrinkage or disappearance, facilitating more effective and less invasive surgery.
2. Tumour Downstaging: Preoperative therapy reduces tumour size and stage, simplifying surgical removal and potentially avoiding extensive procedures like permanent colostomy.
3. Enhanced Local Control: Neoadjuvant therapy reduces the risk of local recurrence by addressing the tumour before surgery.
4. Organ Preservation: In cases of complete clinical response, non-operative management (e.g., "W&W") may be viable, preserving organ function.
5. Better Treatment Adherence: Administering therapy preoperatively may improve adherence to the planned treatment regimen.
Harms
1. Increased Toxicity: Combined chemotherapy and radiation can lead to side effects such as fatigue, gastrointestinal symptoms, skin reactions, and hematologic toxicity.
2. Over-treatment Risk: Some patients may receive unnecessary neoadjuvant therapy, exposing them to treatment risks without added benefit.
3. Risk of Incomplete Response: Delayed surgery due to
neoadjuvant therapy may lead to disease progression or metastasis in nonresponding patients.
Authors Commentary considering other International Guidelines
Potential Advantages of TNT: One of the most notable advantages of TNT is the increased rate of ypCR, where no residual tumour is detected in surgical specimens following treatment with figures reported as being in the region of 30-40%.13,14 Achieving ypCR has been previously associated with improved long-term oncologic outcomes, including better survival rates and reduced recurrence risk.21 Additionally, these therapies contribute to effective tumour downstaging, making previously unresectable or borderline resectable tumours operable and reducing the extent of surgical intervention required. This improved resectability not only facilitates curative surgery but also minimises the need for more invasive procedures.
Enhanced local control is another purported benefit, as preoperative neoadjuvant therapy reduces the likelihood of local recurrence, especially in high-risk tumours with features like positive circumferential resection margins or extramural vascular invasion, although increased rates of local failure was a surprising outcome of the long-term data from the RAPIDO trial.15 Furthermore, in the increased number of patients achieving a cCR, TNT may enable organ-preserving approaches, such as a W&W strategy, potentially avoiding surgical morbidity altogether. Preoperative systemic therapy also improves adherence to planned treatment regimens, as patients are more likely to complete therapy prior to surgery than in a postoperative setting, where recovery and complications may hinder compliance.
Potential Drawbacks of TNT: While TNT has become a cornerstone of treatment for high-risk rectal cancer due to its ability to improve systemic control and ypCR rates, emerging evidence highlights some potential drawbacks that suggest a more nuanced, risk-adaptive approach may be warranted. While TNT has facilitated nonoperative management, W&W and organ-sparing surgery with local excision for patients achieving a cCR or near cCR,7, 8, 22, 23 such strategies carry inherent risks, including local tumour regrowth. The OPRA trial demonstrated higher organ preservation rates
with TNT but also noted increased tumour regrowth compared to opportunistic W&W strategies.7, 8
Approximately 25–30% of patients managed with W&W after TNT experience local regrowth, and salvage surgery is only feasible in 85–90% of cases, and this is may be associated with higher rates of APR and permanent stoma. Moreover, studies indicate that patients experiencing regrowth have significantly higher rates of distant metastases compared to those who undergo upfront surgery.24, 25 Patients with nCR may require prolonged observation to determine if they achieve cCR, delaying definitive surgery.26, 27 This approach can increase surgical complexity due to fibrosis and be associated with increased morbidity and worse specimen quality.28 Such delays may also heighten the risk of local recurrence, particularly in tumours with chemoradiation resistance.
One of the most concerning findings is the increased rate of local recurrence in the long-term follow up of the RAPIDO trial, where the TNT arm (short course radiotherapy and chemotherapy) exhibited a locoregional recurrence rate of 10%, compared to 6% in the CRT group despite improved distant disease free survival.15 There are also reports of a survival paradox where patients achieving ypCR after TNT do not experience improved overall survival compared to those treated with nCRT alone.25 This paradox may reflect the inclusion of tumours with more aggressive biology in TNT protocols, which achieve ypCR or cCR but retain poor longterm prognoses. The notion that the route to ypCR or cCR impacts survival underscores the need for a personalised treatment approach even in such cases.
Any universal application of TNT also risks over-treating patients with low-risk tumours who might achieve optimal outcomes with less aggressive modalities, such as CRT or surgery alone. The PROSPECT trial highlighted the potential for chemotherapy-only approaches in carefully selected low-risk patients, avoiding unnecessary radiation and its associated morbidity. Moreover, there is an increasing realisation that low risk tumours such as those high in the rectum, without a threatened CRM or adverse radiological variables (e.g. mrEMVI or mrTDs). may proceed directly to surgery even with the presence of mesorectal lymphadenopathy. TNT may also intensify treatmentrelated toxicity, particularly hematologic and gastrointestinal side effects. Studies such as RAPIDO and PRODIGE-23 demonstrated higher rates of treatment-associated adverse events compared to standard LCRT alone.14, 16 These toxicities not only compromise quality of life during treatment but may also affect long-term patient recovery. Comparison with other guidelines: Recent National Comprehensive Cancer Network (NCCN) guidelines explicitly recommend immune checkpoint inhibitors for mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) tumors,29 reflecting emerging evidence in this area.30,31 This represents a key divergence from the NCCP guidelines, which, while recommending testing for MSI-H status, do not include specific guidance for immunotherapy. Furthermore, the NCCN strongly advocates for consolidation chemotherapy in TNT strategies, citing superior outcomes from the OPRA trial.

In contrast, the NCCP guidelines allow for more flexibility in treatment sequencing.
The European Society of Medical Oncology (ESMO) guidelines place a particular emphasis on individualised risk stratification using high-resolution MRI to assess key prognostic factors, including CRM involvement, EMVI and lateral lymph node involvement.32 This detailed stratification enables tailored treatment decisions, such as short-course preoperative radiotherapy (SCPRT) or conventional LCRT based on MRI findings. While both NCCP and ESMO advocate for TNT in highrisk cases, ESMO places greater emphasis on CRM involvement and nodal clearance as critical determinants for preoperative strategy. Similarly, both guidelines support NOM in patients achieving a cCR. However, ESMO offers more detailed guidance on intensive surveillance strategies, including MRI and endoscopy intervals, to monitor for tumour regrowth. Notably, while ESMO also highlights the importance of identifying MSI-H status, it does not explicitly recommend immunotherapy for these tumours similar to the recent NCCP document.
Despite their differences, all three guidelines emphasise the centrality of multidisciplinary decision-making, evidence-based stratification, and the need for individualised treatment planning.
Concluding Remarks:
In summary the 2024 Update to the NCCP guidelines for the diagnosis, staging and management of rectal cancer represents the significant evolution in the management of LARC in

Ireland. The adoption of TNT and nCT reflects a paradigm shift toward more personalised, multidisciplinary care. These approaches offer the potential for enhanced tumour downstaging, reduced distant recurrence rates, and organ preservation, ensuring that treatment aligns with both clinical objectives and patient preferences, particularly for high risk rectal tumours.
However, to mitigate the challenges outlined above, a risk-adaptive approach to TNT is suggested. Correct patient selection using established clinical and radiological criteria, particularly MRI-defined criteria, such as CRM involvement and adverse MRI features, should guide TNT application. Baseline evaluation should be performed to exclude high-risk patients unlikely to benefit from W&W, and who should undergo TME regardless of tumour regression grade. Timely response assessment should occur with scheduled surgery for those with poor or partial responses, while patients should be made aware of both the risks as well as the advantages of organ preserving approaches. Limiting the duration of consolidation chemotherapy to 12 weeks and the interval to surgery to 16-20 weeks would mitigate the risk of progression in chemoradioresistant tumours.33 In contrast low-risk patients may benefit from deescalated regimens, such as CRT, chemotherapy-only protocols or direct to surgery.34, 35
In future the use of biomarkers ctDNA and mid-treatment or sequential imaging could better stratify patients based on tumour biology and response.36, 37 Future guidelines should also address the management of those with an nCR including extended surveillance to monitor conversion to cCR, balanced against the risk of delaying surgery.38, 39 In the interim, MDTs will need to consider several complexities outside the scope of the guidelines. These include nuanced decisions about organ preservation and salvage surgery, strategies to avoid overtreatment, and the role of immunotherapy in MSI-H tumours. Additionally, MDTs must evaluate the use of TNT as a primary treatment for early-stage rectal cancer where achieving a cCR and organ preservation is a pre-specified patient treatment goal, even in cases where neoadjuvant therapy would not typically be considered due to the disease’s early stage.
References available on request

Written by Dr Sinead Toomey
1Department of Medicine, RCSI University of Medicine and Health Sciences, 2Beaumont RCSI Cancer Centre


Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and second leading cause of cancer-related mortality worldwide. In 2020 alone, over 1.9 million new cases and nearly 935,000 deaths were reported globally and the global burden of CRC is projected to rise by 60% by 2030.1 Risk factors for CRC include both lifestyle and genetic factors. Modifiable risks include diets high in red or processed meats, low physical activity, obesity, smoking, and excessive alcohol use. Family history, genetic conditions like Lynch syndrome or familial adenomatous polyposis (FAP), and inflammatory bowel diseases also increase susceptibility.
Current treatment strategies for CRC are multidisciplinary and depend on the stage and molecular characteristics of the disease. Surgical resection is the mainstay of treatment for localised CRC and patients are often subsequently given adjuvant chemotherapy to reduce recurrence risk. For rectal cancer, radiotherapy is particularly effective and neoadjuvant chemoradiotherapy is often used to shrink tumours and improve surgical outcomes. Advanced or metastatic CRC is usually treated with systemic therapies, including chemotherapy (e.g. FOLFOX, FOLFIRI), targeted agents such as EGFR inhibitors (e.g. cetuximab, panitumumab), and
angiogenesis inhibitors such as bevacizumab. However, resistance to chemotherapy and targeted therapies ultimately occurs in most patients with advanced disease and subsequent treatment lines offer limited clinical benefit. Furthermore, current treatment modalities often compromise patient quality of life and do not address tumour heterogeneity, a hallmark of CRC, necessitating a more personalised approach to therapy.
In this article we will highlight emerging treatments that address these unmet needs, focusing on novel molecular targets, immunotherapies, and innovative drug delivery systems. These advancements hold promise for improving outcomes and advancing toward precision medicine in the management of CRC.
Large-scale genomic sequencing studies have provided insight into the molecular landscape of CRC. Key mutations frequently found in CRC include APC, TP53, KRAS, BRAF and PIK3CA. The APC gene encodes a tumour suppressor protein that regulates the WNT signalling pathway, which controls cell proliferation, differentiation, and apoptosis. Mutations in APC disrupt this regulatory function, thus driving tumorigenesis. KRAS and BRAF mutations further enhance tumour progression, while loss of TP53 loss results in genomic instability. Mutations in PIK3CA
activate the PI3K/AKT pathway which promotes cell survival, proliferation, and resistance to apoptosis. In addition, PIK3CA mutations frequently co-occur with other mutations, including mutations in KRAS or APC, augmenting oncogenic effects. Epigenetic modifications, including DNA methylation and histone acetylation, contribute to gene silencing and tumour evolution, underscoring the complexity of CRC biology. In recent years, efforts to target these molecular aberrations have yielded significant advancements in CRC treatment.
Epidermal growth factor receptor (EGFR) inhibitors, such as cetuximab and panitumumab, are effective in treating left-sided CRC patients with wild-type KRAS and NRAS genes. These monoclonal antibodies block EGFR-mediated signalling pathways that promote cell proliferation and survival. In metastatic CRC, EGFR inhibitors have prolonged overall survival to over 24 months. However, resistance develops in most patients, and their effectiveness is significantly reduced in cases of KRAS wild-type right-sided tumours or tumours harbouring KRAS or NRAS mutations, emphasizing the need for molecular stratification prior to treatment.
The vascular endothelial growth factor (VEGF) pathway plays a central role in tumour angiogenesis. Bevacizumab, a monoclonal antibody that targets VEGF, has been shown extend overall survival up to 17.7 months when combined with chemotherapy in metastatic CRC. Other newer agents, such as aflibercept and regorafenib, also target VEGF-related pathways, and provide additional options for antiangiogenic therapy.
Mutations in BRAF and KRAS occur in approximately 10% and 40% of CRCs, respectively and are linked to a poorer prognosis. The BRAF inhibitor encorafenib in combination with cetuximab has shown clinical efficacy in CRCs with BRAF V600E mutations. Recent advancements in targeting KRAS, previously
considered undruggable, include the development of KRAS G12C inhibitors such as sotorasib and adagrasib. Although KRAS G12C mutations have shown potential for targeted therapy with sotorasib in lung cancer, resistance to this treatment emerges rapidly and to date, no clinically approved inhibitors are currently available for KRAS G12D, the most common KRAS mutation observed in CRCs. Emerging Biomarkers
Biomarkers such as HER2 amplification and mutations in PIK3CA are expanding the landscape of targeted therapies in CRC. Dual anti-HER2 therapy has been shown to have clinically meaningful anti-tumour activity in patients with HER2-positive metastatic CRC, while there are ongoing trials of the PIK3CA inhibitor alpelisib. This further highlights the importance of molecular profiling in guiding treatment for patients with advanced CRC.
Immunotherapy Advances
Immunotherapy has transformed the treatment landscape for various cancers, including CRC. By leveraging the immune system to target and eliminate cancer cells, immunotherapeutic approaches are increasingly recognised for their potential in CRC, particularly in specific molecular and immunological subtypes. Microsatellite Instability (MSI) and Mismatch Repair Deficiency (dMMR) are predictive biomarkers for immunotherapy response. High microsatellite instability (MSI-H) tumours, which are characterised by defective DNA mismatch repair, have a high mutational burden and produce high levels of neoantigens, making them highly immunogenic and responsive to immune checkpoint inhibitors.
Programmed death-1 (PD-1) and its ligand, PD-L1, are immune checkpoint molecules that inhibit T-cell activation and allow tumours to evade immune surveillance. Antibodies targeting these proteins, such as pembrolizumab and nivolumab, block this interaction, restoring T-cell activity



Figure 1: Treatment strategies for colorectal cancer

and promoting anti-tumour immunity. Pembrolizumab is FDAapproved for CRC patients with MSI-H or dMMR, which account for approximately 15% of all CRC cases. Clinical trials have shown durable responses and improved survival in these patients, making pembrolizumab a first-line treatment for advanced MSI-H/ dMMR CRC.
Cytotoxic T-lymphocyteassociated antigen 4 (CTLA-4) is another immune checkpoint that inhibits T-cell activation. The combination of nivolumab and the CTLA-4 inhibitor ipilimumab has significantly improved outcomes in MSI-H metastatic CRC, demonstrating durable clinical benefits over more than four years of follow-up with high response rates, low disease progression rates, and improved long-term survival. In the non-metastatic setting, the recently published NICHE-2 study reported remarkable results with this combination in patients with locally advanced dMMR colon cancer. After just four weeks of treatment, pathological responses were observed in 98% of patients, with 95% achieving a major pathological response and 68% achieving a pathological complete response.
While chimeric antigen receptor (CAR)-T cell therapy has been highly effective in haematological malignancies, its application in CRC and other solid tumours has been limited by challenges such

of proteomic profiling could provide insights into resistance mechanisms and uncover potential novel therapeutic targets, while metabolomic profiling could reveal metabolic vulnerabilities which could be exploited for treatment.

as the immunosuppressive tumour microenvironment and the lack of ideal targets. However, targets such as CEA and EGFR have shown promise in early-phase trials, and ongoing research aims to overcome the barriers posed by the immunosuppressive tumour microenvironment in CRC.
Precision Oncology and Personalised Medicine
Precision oncology and personalised medicine have the potential to revolutionise CRC treatment by tailoring interventions to the unique molecular and genetic profiles of each patient. Advances in diagnostic technologies and computational methods have enabled real-time monitoring, individualised treatment selection, and data-driven decision-making, offering the potential to improve outcomes while minimizing unnecessary toxicity.
Liquid biopsies, which analyse circulating tumour DNA (ctDNA) and other biomarkers in blood, provide a non-invasive method for monitoring CRC progression and treatment response and provide a dynamic picture of tumour evolution. ctDNA analysis could potentially facilitate the detection of minimal residual disease (MRD) after surgery, guiding the need for adjuvant therapy. Additionally, it could be used to identify emerging resistance mutations during treatment, enabling timely adjustments in therapy.
The incorporation of omics technologies into clinical care allows for comprehensive tumour profiling. Genomic sequencing can be used to identify actionable mutations to guide the use of targeted therapies and immunotherapies. The addition
In research, artificial intelligence (AI) driven tools are increasingly employed to analyse complex omics datasets. These tools can predict treatment responses based on molecular profiles, optimise drug combinations, and identify novel biomarkers. Despite challenges related to data standardisation, interpretability, and integration into clinical workflows, AI-driven tools have the potential to enable truly personalised care. This approach could lead to improved patient outcomes and more efficient utilisation of healthcare resources.
Traditional systemic delivery methods often fail to achieve optimal therapeutic concentrations in tumours and can be associated with significant toxicity. Novel drug delivery approaches have the potential to facilitate targeted drug delivery, provide controlled release, penetrate tumour microenvironments, preserve drug stability, and reduce systemic toxicity.
The use of nanoparticles (NPs) to encapsulate chemotherapeutic agents or molecularly targeted agents can enhance bioavailability and improve drug stability. Clinical trials have shown that liposomal formulations of chemotherapeutic agents, such as irinotecan, have improved efficacy and reduced toxicity.
Antibody-drug conjugates (ADCs), which combine monoclonal antibodies with cytotoxic agents to deliver targeted therapy to the tumour, are another promising strategy. ADCs targeting HER2 (trastuzumab deruxtecan) and Trop-2 (sacituzumab govitecan) have shown promising early results in patients with advanced stage CRC.
Combination therapies in cancer treatment can enhance efficacy by targeting multiple pathways or mechanisms involved in tumour progression. This strategy not only improves treatment outcomes but also allows for the use of lower doses of individual agents, reducing toxicity and side effects for patients. Additionally, combination therapies can help overcome resistance, as cancer cells are less likely to evade
multiple concurrent mechanisms of action. For example, dual inhibition of MEK and BRAF may potentially address resistance in BRAFmutant CRC.
The combination of EGFR or VEGF inhibitors with immune checkpoint inhibitors is also being investigated as a strategy to modulate the tumour microenvironment and increase its susceptibility to immune-mediated destruction. The NRG-GI004/SWOG S1610 clinical trial is exploring the synergy between bevacizumab (an antiVEGF agent) and atezolizumab (an anti-PD-L1 antibody) in metastatic CRC, aiming to harness the antiangiogenic effects of bevacizumab to boost immune responses.
Recent pre-clinical studies from our lab have demonstrated that dual targeting of PI3K and the cell cycle using a CDK4/6 inhibitor exhibits potent anti-proliferative effects and significantly enhanced anti-tumour activity compared to single agent treatments.2,3 This combination may be a promising novel therapeutic strategy for colorectal cancer and warrants further clinical investigation.
Conclusion
Treatment for colorectal cancer has seen remarkable advancements, particularly in molecularly targeted therapies, immunotherapy, and precision medicine. The integration of immune checkpoint inhibitors, novel biomarkers, and real-time monitoring technologies has significantly improved outcomes for select patient populations, such as those with MSI-H/dMMR tumours or actionable mutations like BRAF or EGFR.
Despite these advancements, challenges remain. Resistance mechanisms, high costs, and the variability of patient responses pose barriers to widespread implementation. Additionally, many promising approaches, such as CAR-T therapy or multiomics integration, require further validation in large-scale clinical trials before being incorporated into routine clinical practice.
Looking forward, the future of CRC treatment lies in combining therapies tailored to individual tumour profiles, leveraging realtime monitoring technologies, and incorporating artificial intelligence for data-driven decision-making. By addressing existing challenges and fostering multidisciplinary collaboration, these innovations will pave the way for more personalised and effective care, and ultimately improve outcomes for patients with CRC.
References available on request


Testicular cancer, by nature, is one of the most aggressive solid organ tumours. It can spread to virtually every part of the body but commonly to lymph nodes, lungs, and brain. If left untreated, it will cause death in the majority of patients within the first year of diagnosis. Treatment involves a multi-modality approach utilising chemotherapy, radiotherapy, and surgery. Since the birth of cisplatin-based chemotherapies in the 1970s, a cure can be achieved in more than 90% of cases. This high cure rate for around 50 years has resulted in a significant number of survivors. As testicular cancer is commonly diagnosed in the early years of life, these survivors live long enough to experience the late side effects of treatment, which are not observed in other patient groups.
Written by Dr M Raheel Khan, Consultant Medical Oncologist, St James’s Hospital, Dublin
As early as the early 90s, oncologists started observing the late side effects in this population, but in the last decade, we have seen an exponential amount of research in this area. Of note, a registry-based research performed on Scandinavian, American, and Canadian subjects revealed a worrisome picture of this particular cohort. The study showed a downward trend in life expectancy of testicular cancer survivors while it was improving in the general population for the last five decades. Further investigations showed second cancers as the most common cause of death, followed by early cardiovascular disease. With these revelations, more research was carried out on the survivors in the last five years. It has been established now that the late side effects are associated with the treatment modalities used for the cure of cancer, in particular, chemotherapy and radiotherapy, being the major culprits. In addition to early deaths, these sequelae cause excessive disease burden in the survivors, ultimately affecting their economic, social, and sexual health. Other side effects commonly reported in
this cohort include hypertension, dyslipidaemia, hypogonadism, obesity, lung disease, kidney disease, deafness, tinnitus, peripheral neuropathy, and infertility. Fear of cancer recurrence and anxiety are the most common psychological consequences in the survivors.
Radiotherapy to abdominal lymph nodes results in a higher incidence of abdominal malignancies, including stomach, pancreas, bowels, and prostate cancer. Therefore, it is now only being used in exceptional circumstances. It is still routinely used to treat the brain mets, which can cause cognitive decline in survivors.
Cisplatin-based chemotherapy regimens are still widely used for the treatment of testicular cancer. Plenty of efforts have been made to reduce its exposure in the last decade. Firstly, its use in the stage I disease after orchiectomy is almost abandoned, with preference being given to surveillance only. For stage II disease, with cancer present in abdominal lymph nodes, surgical resection without chemotherapy is widely being investigated and discussed at the moment. This approach, however, requires highly skilled and specialised surgical teams performing surgeries in high-volume cancer centres. Chemotherapy remains the only option in stage III disease except for a small number of cancers which do not respond to chemotherapy and require surgery.
Reducing the exposure to chemotherapy and radiotherapy will definitely improve the incidence of late side effects in survivors. But there is a dire need to address the challenges being faced by the patients who are exposed to these treatments in the past. Unfortunately, our progress in survivorship care has not been satisfactory so far, marked by the absence of any specialised pathways and services.
As an oncology team in Tallaght University Hospital, Dublin, we acknowledged this dire need for specialised services and established a nurse-led clinic for testicular cancer survivors. A similar clinic is recently developed in St James’s Hospital, Dublin, while work is in progress to include more cancer centres. More than 100 patients were screened by our team to assess the prevalence of mortality and morbidity in Irish testicular cancer survivors. Furthermore, we laid down the basis of a national prospective registry for survivors to study the trends in this population. This study, SLECT, is being conducted in collaboration with Cancer Trials Ireland and has been actively recruiting for the last 2 years. In an effort to improve the health of survivors, referral pathways and services are being set up, specifically designed for this cohort. While we have been successful in many aspects, a big hiatus in service delivery remains, and the work continues.
A new framework to support the safe and effective use of robotic-assisted surgery has been published today by the Royal College of Surgeons in Ireland (RCSI). As robotic technology continues to revolutionise the operating room, this new guide sets the standard for governance, empowering hospitals and surgeons to embrace the future of surgery with confidence while prioritising patient safety.
The comprehensive guide, titled Robotic Surgery Governance in Ireland: A Guide to Good Practice, was published at the opening of Ireland’s first-ever Robotic Learning Village which is taking place during RCSI’s annual Charter Day meeting.
Robotic assisted surgery offers precision, minimal invasiveness, and the potential for faster patient recovery times. These systems are now regularly used for routine and complex procedures across Ireland, making the need for a cohesive national governance framework urgent.
The RCSI guide addresses this gap, offering a structured approach to the adoption of robotic surgery that will enable healthcare institutions and surgeons to confidently navigate the rapidly evolving landscape of surgical technology. It provides clear, actionable recommendations for hospitals to integrate robotic surgery programmes, focusing on training, credentialing, and governance to ensure patient safety and enhance surgical outcomes.
Professor Deborah McNamara, President of RCSI, said: “At RCSI, we are committed to supporting the professional development of surgeons while ensuring that the latest technological advancements are used responsibly. Our new guide is aimed at ensuring that hospitals and surgeons have the structured support they need to implement this cutting-edge technology while maintaining the highest standards of patient care and safety.”
“This framework is not just a set of guidelines – it’s a vital tool for surgeons and hospitals to maximise the potential of robotic surgery while minimising risk,” explained Professor Barry McGuire, RCSI Professor of Postgraduate Surgical Education and Academic Development, and lead author of the guide. “Drawing from lessons learned in the early days of laparoscopic surgery, we are proactively addressing the challenges and opportunities posed by robotic platforms, ensuring safety and excellence in patient care.”
Continuing Professional Development
Rheumatoid arthritis (RA) is a common inflammatory arthritis affecting 1% of the population. While joint manifestations may be the most visible issue, it is a true systemic disease affecting multiple organs and systems. Characteristic symptoms include early morning stiffness, and pain and swelling in the small joints of the hands and feet. Interstitial lung disease affects 8% of people with RA and is associated with significant reduced survival. Inflammatory markers (ESR and CRP) are generally elevated in RA, but may be normal.
25% of patients will be negative for both rheumatoid factor and CCP antibody. Many individuals with a positive rheumatoid factor in particular do not have RA.
Methotrexate is the first line treatment and anchor drug for other treatment strategies. A wide range of treatments are now available for RA collectively called disease-modifying antirheumatic drugs (DMARDS). These include conventional synthetic DMARDS such as leflunomide, sulfasalazine, and hydroxychloroquine, biologic DMARDS such as TNF inhibitors, interleukin-6 inhibitors, and B and T cell affecting agents, and the JAK inhibitors.
Combination treatment strategies are generally employed if methotrexate is insufficient. The ability to identify the correct medication for an individual patient a priori remains an elusive goal.
AUTHOR: by Professor Richard Conway, Consultant Rheumatologist, St. James Hospital; Clinical Associate Professor, Trinity College Dublin Richard Conway graduated from the Royal College of Surgeons in Ireland in 2006. He completed rheumatology and general internal medicine training in Ireland in 2014, and a PhD in giant cell arteritis at University College Dublin in 2017. He is currently a consultant rheumatologist and physician at St. James’s Hospital and a clinical associate professor at Trinity College Dublin. He is the author of more than 200 peer-reviewed publications and 4 book chapters. His research interests include interstitial lung disease in systemic rheumatic diseases, polymyalgia rheumatica, and systemic vasculitis.
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result? Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the

4 previous steps, log and record your findings.
Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
A 32 year old female presented with a 3 month history of pain and swelling in her hands and feet. She reports a progressive increase in symptom severity. Initially they were stiff and sore for the initial hour after rising in the morning but now this has extended through until the afternoon. On examination she has tender swollen metacarpophalangeal and proximal interphalangeal joints in her hands and is sore and swollen across the balls of both feet. Initial bloods tests show an elevated C-reactive protein of 31 mg/L and erythrocyte sedimentation rate of 45 mm/hr. She has positive rheumatoid factor of 65 IU/ml and anti-cyclic citrullinated peptide antibodies >340. Plain radiographs of her hands and feet are reported as normal. She is diagnosed with seropositive rheumatoid arthritis and commenced on treatment with methotrexate 10mg a week and folic acid 5mg a week. She was also prescribed a prednisolone taper of 20mg daily for a week, 15mg daily for a week, 10mg daily for a week, 5mg daily for a week, and then stopping. She was referred to a clinical nurse specialist for disease and medication education, and to an occupational therapist for a joint protection programme. A review was planned for 6 weeks time.
Rheumatoid arthritis is a chronic systemic inflammatory disease affecting 1% of the population of the developed world. It is 2-3 times as common in women than men. Joint manifestations are frequently the first and most evident symptom; however it is a true systemic inflammatory condition affecting multiple organs. Of these an increase in cardiovascular disease equivalent to that seen with type 2 diabetes mellitus, and the occurrence of interstitial lung disease in 8% of patients represent the most significant problems. Due mainly to these factors, untreated rheumatoid arthritis is associated with a significant increase in mortality. Appropriate treatment decreases this risk.
Rheumatoid arthritis has classically been described as a symmetrical deforming polyarthritis. In modern rheumatology practice this classical aphorism no longer holds true. Our goal is to diagnose and treat rheumatoid arthritis as early and aggressively as needed to eliminate symptoms. It is distinctly unusual in modern rheumatology to see a patient diagnosed since the advent
of biologic therapies develop significant deformities.
In the early stages of rheumatoid arthritis the disease is not always symmetrical nor affecting a large number of joints and this will only develop with untreated progressive disease, a failure of treatment. New onset rheumatoid arthritis therefore can present as an oligo or even mono-arthritis, and may be asymmetrical.
Joint pain and swelling is the cardinal feature of rheumatoid arthritis, but can also be caused by a multitude of other conditions. In rheumatoid arthritis the small joints of the hands and feet are characteristically affected but large joints and the cervical spine may also be involved. The lower back and distal interphalangeal joints in the hands are classically spared in rheumatoid arthritis. Joint stiffness on first walking in the morning (early morning stiffness) is characteristic of inflammatory arthritis, this typically also improves with activity. All of us can be a little creaky on first waking, a duration of at least 30 minutes is normally considered significant. Patients with osteoarthritis consistently do also have early morning stiffness, but with a duration of minutes rather than hours. A patient with hand osteoarthritis will typically
identical, in the early stages. The diagnosis of inflammatory arthritis as a collective group, or any of the individual conditions, is made by clinician gestalt. There are no diagnostic criteria or definitive tests available. A number of different classification criteria have been published for these diseases; the purpose of these is to provide a homogenous patient cohort for research purposes, they perform poorly when used for diagnosis and should not be used for such.
report being worse first thing in the morning and it is crucial to elucidate the specific duration before attributing this to an inflammatory arthritis.
The key clinical examination finding of rheumatoid arthritis is synovitis, a boggy, usually (but not always) tender, swelling in the joints. This is distinct from the hard bony swelling (nodes) seen in osteoarthritis. Due to the multi-system nature of the disease patients may also present with symptoms from other organs, the most common of these are dry gritty eyes, exertional dyspnoea and dry cough.
Given that our aim is to diagnose and treat rheumatoid arthritis before the development of its textbook manifestations, the question then becomes how does one diagnosis early rheumatoid arthritis and differentiate it from other types of arthritis? This may sometimes be difficult, but in fact is often not that important; the key
question is not whether the patient has rheumatoid arthritis or not, but whether they have an inflammatory arthritis or not. Inflammatory arthritis comprises a group of conditions, the most common of which are rheumatoid arthritis, psoriatic arthritis, and reactive arthritis, although there are many others. Importantly, the treatment of these is broadly similar, if not identical, in the early stages. The diagnosis of inflammatory arthritis as a collective group, or any of the individual conditions, is made by clinician gestalt. There are no diagnostic criteria or definitive tests available. A number of different classification criteria have been published for these diseases; the purpose of these is to provide a homogenous patient cohort for research purposes, they perform poorly when used for diagnosis and should not be used for such.
A number of factors increase the likelihood of a patient having inflammatory arthritis and are factored into the clinical diagnosis. Early morning stiffness for greater
than 30 minutes is a strong indicator of an inflammatory condition. The presence of synovitis (soft boggy swelling) is a good predictor but requires experience to appreciate, especially in subtle cases. Elevated acute phase reactants (C-reactive protein, erythrocyte sedimentation rate) do increase the likelihood of an inflammatory arthritis but should not be relied on as they can be normal in the presence of rheumatoid arthritis, and indeed elevated in its absence. Radiographic erosions, while typically seen in advanced rheumatoid arthritis, should not commonly be encountered in modern presentations. Rheumatoid factor and anti-cyclic citrullinated peptide antibodies are not useful in diagnosing inflammatory arthritis. They are useful in characterising a patient with inflammatory arthritis as seropositive rheumatoid arthritis. However, if they are used in unselected patients with joint pain they are more likely than not to reveal false positives.
Many textbooks still place nonsteroidal anti-inflammatory drugs (NSAIDs) as the first line treatment of rheumatoid arthritis. This is incorrect. These drugs provide some symptomatic relief but are insufficient for the vast majority of patients with rheumatoid arthritis and risk delaying the institution of definitive effective treatment. They are also associated with common, potentially severe adverse events when used chronically (and rheumatoid arthritis is a chronic incurable condition).
A number of factors increase the likelihood of a patient having inflammatory arthritis and are factored into the clinical diagnosis. Early morning stiffness for greater than 30 minutes is a strong indicator of an inflammatory condition. The presence of synovitis (soft boggy swelling) is a good predictor but requires experience to appreciate, especially in subtle cases. Elevated acute phase reactants (Creactive protein, erythrocyte sedimentation rate) do increase the likelihood of an inflammatory arthritis but should not be relied on as they can be normal in the presence of rheumatoid arthritis, and indeed elevated in its absence. Radiographic erosions, while typically seen in advanced rheumatoid arthritis, should not commonly be encountered in modern presentations. Rheumatoid factor and anti-cyclic citrullinated peptide antibodies are not useful in diagnosing inflammatory arthritis. They are useful in characterising a patient with inflammatory arthritis as seropositive rheumatoid arthritis. However, if they are used in unselected patients with joint pain they are more likely than not to reveal false positives.
Methotrexate is the first line and anchor drug (in combination strategies) in rheumatoid arthritis. Its regularly attributed mode of action of folate antagonism is too simplistic and we do not fully understand its effect in rheumatoid arthritis. It is however remarkably effective. Concerns have existed over potential adverse events, however high quality research studies in recent years have shown that these are largely unfounded, and that methotrexate is a far
Musculoskeletal Pain
Local muscular or tendinopathy
Treatment
Many textbooks still place non-steroidal anti-inflammatory drugs (NSAIDs) as the first line treatment of rheumatoid arthritis. This is incorrect. These drugs provide some symptomatic relief but are insufficient for the vast majority of patients with rheumatoid arthritis and risk delaying the institution
and then stop.
safer treatment than NSAIDs or glucocorticoids. Indeed, methotrexate treatment is far safer than having active untreated rheumatoid arthritis; rheumatoid arthritis is associated with a significant increase in mortality above the general population. Methotrexate use is associated with a 70% reduction in mortality in rheumatoid arthritis. The starting dose of methotrexate is 10 to 15mg once a week, this is combined with folic acid 5mg at least once a week to prevent adverse events. The dose can be up-titrated at 3-monthly intervals until control is achieved, for most patients a weekly dose of 20-25mg is not exceeded. Methotrexate is most commonly initially prescribed orally but subcutaneous formulations have the potential for better bioavailability and reduced adverse events.

A number of other diseasemodifying anti-rheumatic drugs (DMARDs) are also used. Commonly used DMARDs include sulfasalazine, hydroxychloroquine, and leflunomide. These can be utilised either instead or in combination with methotrexate. Triple therapy, consisting of methotrexate, sulfasalazine, and hydroxychloroquine appears to have efficacy close to that of the more expensive biologic agents.
If patients continue to experience significant symptoms despite DMARD treatment the usual next step is to add in a biologic medication. In a general sense, these agents work by targeting a specific component of the aberrant immune-inflammatory response in rheumatoid arthritis. Because they are specifically designed medications, they less frequently cause adverse events than traditional DMARDs, albeit when
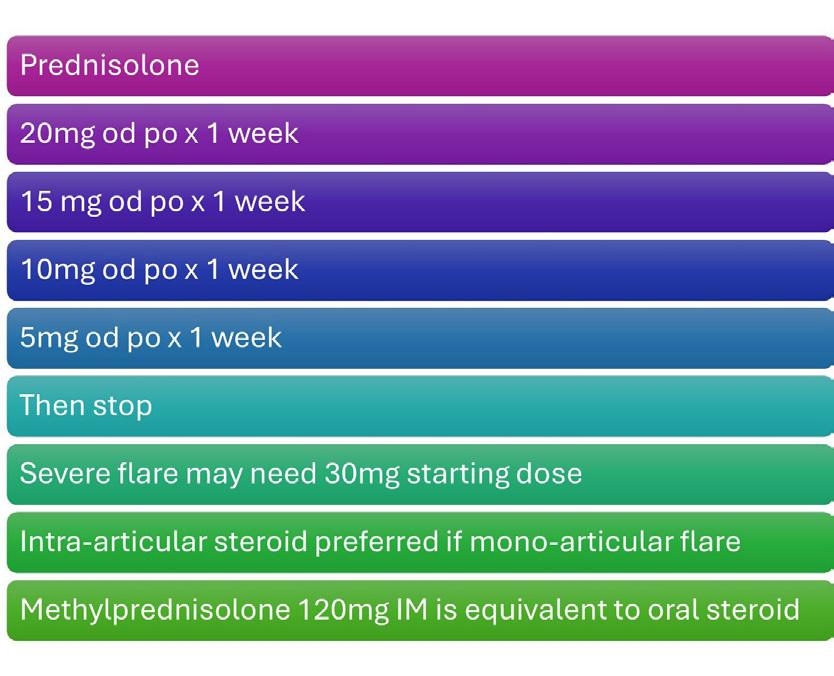
It is very likely that new treatments for rheumatoid arthritis, especially biologic agents and biosimilars, will continue to enter the market. It is less likely that any of these will prove to be significantly more efficacious than others. This is due to the inherent complexities of rheumatoid arthritis. It is increasingly recognised that rheumatoid arthritis is not one “disease”, rather it is a heterogenous mix of related conditions with a similar phenotype. The molecular basis for the initiation and continuation
these (predominantly infection related) adverse events occur they are more serious. There are multiple agents now available; these include tumour necrosis factor inhibitors (etanercept, adalimumab, infliximab, golilumab, certolizumab), interleukin-6 inhibitors (tocilizumab), B-cell depleting agents (rituximab), T-cell modulators (abatacept), and janus kinase inhibitors (tofacitinib, baricitinib, upadacitinib, filgotinib). Less expensive (but equally effective) equivalents of the originator biologic agents, termed biosimilars, are now available. All of these agents require careful pre-assessment for latent infections (tuberculosis, viral hepatitis) and other contraindications and cautions prior to their use. These agents are life-changing for many patients, but must be started and monitored in the appropriate setting.
All of the available DMARD and biologic treatments for rheumatoid arthritis take a number of months for a full effect to be seen; we do not evaluate treatment response until after 3 months for this reason. Rheumatoid arthritis is frequently a profoundly symptomatic condition and patients may suffer significantly while waiting for treatment to take effect. This is where there is a role for glucocorticoid treatment in the short term. Glucocorticoids are a remarkably effective treatment for rheumatoid arthritis but associated with significant adverse events, particularly in the long term. A short course of glucocorticoids (steroid taper), however is generally safe and can dramatically improve a patient’s
symptoms. There are many ways of utilising this steroid taper, the author’s preference is to give 20mg prednisolone daily for a week, 15mg daily for a week, 10mg daily for a week, 5mg daily for a week, and then stop.
Future treatments
It is very likely that new treatments for rheumatoid arthritis, especially biologic agents and biosimilars, will continue to enter the market. It is less likely that any of these will prove to be significantly more efficacious than others. This is due to the inherent complexities of rheumatoid arthritis. It is increasingly recognised that rheumatoid arthritis is not one “disease”, rather it is a heterogenous mix of related conditions with a similar phenotype. The molecular basis for the initiation and continuation of inflammation is different for different groups of patients with the disease. Even within the same patient, multiple cytokines are involved in the inflammatory process, to a greater or lesser extent. The clinical implication of this is that in each individual patient some medications, and particularly biologic agents, are more likely to work than others. At present we are essentially unable to identify which agent is most likely to work a priori. The ability to do so is the holy grail of treatment in rheumatoid arthritis, termed personalised medicine. Extensive efforts have been underway in this field for a number of years with no great progress to date. Again, this likely relates to the complex and heterogenous nature of the disease. It is hoped that this will change in the future and we will enter an era where we can offer patients an initial treatment for “their” rheumatoid arthritis.
Interstitial Lung Disease in Rheumatoid Arthritis
Pulmonary disease is the second most cause of mortality in RA. While a plethora of pulmonary manifestations have been described in RA the most serious, and unfortunately one of the more frequent is RA related interstitial lung disease (RA-ILD).
The burden of RA-ILD has been difficult to fully ascertain. The seminal cohort study reported a lifetime risk of 7.7% for ILD in RA
patients compared to 0.9% for ILD in the general population. More recent studies have reported the development of incident RA-ILD in 3-5% of RA patients over shorter follow up periods. All of these studies evaluated symptomatic RA-ILD, which is the key clinically relevant question. Screening for RA-ILD in asymptomatic patients reveals a far greater prevalence. Exact figures depend on the methodology and diagnostic thresholds used but can be up to 67% of RA patients screened with CT thorax and 80% of those histologically screened. It would not be an unreasonable interpretation of these numbers to suggest that all RA patients have some degree of ILD if one looks hard enough, again reflecting the true systemic nature of the disease process. The clinical relevance of the identification of such asymptomatic cases is less certain, however.
A number of key features of RA development suggest that the lung may be a primary site of the initiation and propagation of RA. These include the association with smoking, the pulmonary manifestations of RA, and the finding of citrullinated antibodies in early and pre-RA disease. Identified risk factors for the development and subsequent severity of RA-ILD include age, male sex, disease activity, smoking, and seropositivity for rheumatoid factor or anti-cyclic citrullinated peptide antibodies. The gain-of-function MUC5B promoter variant rs35705950 which was first described in IPF is also associated with the development of RA-ILD.
While generally occurring in a patient with known RA, RA-ILD may also occur in patients with joint pain but no formal diagnosis of RA, and more less commonly as the first manifestation of rheumatoid disease in a patient with no prior or contemporary joint based symptoms. RA-ILD may be clinically silent for many years and most commonly presents with an insidious onset of symptoms. This can be further exacerbated by the functional limitations of the articular disease limiting recognition of incipient exertional dyspnoea. More rarely it can present as a fulminant ILD process reminiscent of the Hamman-Rich
syndrome. The characteristic presentation of RA-ILD is with slowly progressive exertional dyspnoea and dry cough. Fine bibasal crepitations on lung examination and finger clubbing are common but are generally not identifiable until later in the disease course. The radiologic findings of RA-ILD most commonly resemble IPF with a usual interstitial pneumonia (UIP) most common, other patterns such as non-specific interstitial pneumonia (NSIP) can occasionally be seen. The optimum management of RAILD remains uncertain. It makes intuitive sense that controlling the rheumatoid disease process itself would be a central pillar of any management strategy. There is good evidence that increased RA disease activity is related to the development and progression of RA-ILD. One of the difficulties we have in ascertaining RA disease control is that all of our existing measures are heavily weighted towards arthritis measures. Our contemporary understanding of RA as a pansystemic disease implies that we require measures encompassing non-articular disease activity measures to ensure appropriate disease control in all patients. Methotrexate is our first line and anchor drug for the management of rheumatoid arthritis. The previous implication of methotrexate as a contributory agent to the development of RAILD has been comprehensively shown to be erroneous.
Methotrexate is associated with a very rare acute pneumonitis but this is entirely distinct from RA-ILD. In fact, methotrexate appears to be protective for both the development and progression of RA-ILD, mirroring its efficacy in articular disease. Our evidence for the use of specific biologic disease-modifying antirheumatic drugs, which have become central to the management of severe RA, is lacking due to a dearth of randomised controlled trials. There is some evidence that rituximab and abatacept, rather than TNF-inhibitors, may be the agents of choice in the setting of RA-ILD. The use of these agents in observational studies has been reported to halt disease progression in the majority of treated patients. The evidence to support this remains limited,
however given their equivalent efficacy in RA generally it appears reasonable to use them as the biologics of choice in RA patients with ILD. A complementary strand to management has been opened with the advent of anti-fibrotic agents, with nintedanib being shown to have equivalent efficacy in RA-ILD as was previously demonstrated in idiopathic pulmonary fibrosis.
The natural history of clinically relevant symptomatic RA-ILD appears to be one of inexorable progression. The median survival from diagnosis in historic cohorts is 2.6 years. The natural history of asymptomatic RA-ILD appears to be frequently more benign and may not progress in many patients, although this requires further confirmation. Observational study evidence of abatacept and rituximab suggests that these agents may be able to arrest RA-ILD disease progression in the majority of patients. Anti-fibrotic agents appear to be associated with continued disease progression but they decelerate this evolution. Pulmonary fibrosis is the end stage result of the inflammatory pulmonary process inherent in RA. As a general rule, established pulmonary fibrotic change is not readily reversible. It is theorised that early aggressive intervention encompassing complete RA disease activity suppression, perhaps in combination with specific therapeutic agents may lead to greatly improved outcomes by intervening prior to the development of this irreversible lung damage. Such a strategy while theoretically appealing, requires to be proven.
Rheumatoid arthritis is a common severe systemic inflammatory disease. Early diagnosis and prompt treatment are key to improving patient’s symptoms and prognosis. Early institution of methotrexate treatment with aggressive treatment escalation to achieve disease remission achieves optimum outcomes. In those with severe rheumatoid arthritis, the ability to predict which biologic agent is best for each individual is a crucial goal of current research.



Written by Jemma Buchalter, Michaela J. Higgins


What is hormone receptorpositive breast cancer?
Breast cancer subtypes can be characterised by the expression of oestrogen, progesterone and human epidermal growth factor (HER2) receptors on the surface of tumour cells. The identification of these subtypes plays a crucial role in selection of the optimal treatment plan for a patient with breast cancer. The most common subtype of breast cancer is hormonereceptor-positive (HR+), HER2negative breast cancer accounting for approximately 70% of female breast cancer cases world-wide. HR+/ HER2- breast cancer also carries the best prognosis with a 5-year relative survival rate of approximately 95.1%. Treatments that target hormone receptors in both the early and advanced settings can provide excellent clinical outcomes for patients and are associated with less toxicity than chemotherapy.1, 2
Surgery is the primary treatment for early breast cancer (i.e. to remove completely disease confined to the breast and/or local lymph nodes). Hormone-blocking treatment (sometimes termed ‘endocrine therapy’), chemotherapy or a combination of both, are recommended after surgery to reduce the risk of recurrence. Radiation may also be provided to the chest wall and draining lymph node regions. This is termed ‘adjuvant therapy’. Choice of treatment/s offered are increasingly tailored based on tumour- and
patient-specific factors including tumour size, receptor status, grade, nodal status, patient co-morbidities and genomic signature. In this article we will focus on adjuvant anti-hormonal treatment of HR+/ HER2- breast cancer, including selection criteria, duration of treatment and common adverse effects of treatment.2,3
In mammary gland development oestrogen receptors (ER) have a critical role in controlling cell division in coordination with other hormones such as progesterone. In tumour cells that express an increased level of ER’s, oestrogen-mediated cell division becomes upregulated leading to tumorigenesis, growth and proliferation of malignant cells. As a result these tumours are sensitive to therapies that block ER activity, such as endocrine therapy.3 The degree of immunohistochemical (IHC) expression of oestrogen and progesterone receptors by tumour cells help predict who will respond best to hormone-blocking endocrine therapy. (hereafter referred to as ET). A breast cancer is classified as ER positive when ≥1% of tumour nuclei stain positive for ER. Tumours with very low (1-10%) ER staining on IHC behave and may be treated like triple negative breast cancers but have been shown to benefit from the addition of adjuvant ET.4, 5
Current standard of care adjuvant ET is with either an aromatase inhibitor (AI) such as anastrozole, letrozole or exemestane or a
selective oestrogen receptor modulator (SERM), such as Tamoxifen. SERMs exert their effect by directly blocking oestrogen receptors and can be used safely and independently in both pre- and post-menopausal women. AI’s act upstream in the oestrogen pathway preventing the conversion of androgens to oestrogen, this form of oestrogen blockade is optimal in post-menopausal women. In premenopausal women a compensatory response is produced when an AI is used via increased ovarian oestrogen production. Therefore, when using an AI in pre-menopausal women it is imperative to administer adequate ovarian function suppression (OFS), such as an gonadotropin-releasing hormone agonist (GnRH), to eliminate this compensation and adequately suppress oestrogen levels.3
Choice of adjuvant endocrine therapy
To determine the appropriate endocrine treatment for patients’ several prognostic factors are considered including receptor status, proliferation index, tumour grade, anatomical stage (size/ nodal burden) and genomic assay with the latter three being the most significant indictors of recurrence risk. For example, a small, nodenegative, low-grade tumour with high ER/PR expression tends to have a better overall prognosis and lower recurrence rate than a larger, high grade, node-positive tumour with lower ER/PR expression.2, 6 Both increasing tumour diameter and larger nodal burden amount to higher rates of recurrence, i.e. a T1N0 tumour would have an expected 5 to 20-year recurrence rate of 13% in contrast to larger and node-positive tumours which have a recurrence risk of higher than 40%.6 Uniquely, the risk of recurrence continues for many years after diagnosis in ER-positive breast cancer.6
A pivotal meta-analysis from the Early Breast Cancer Trials Collaborative Group (EBCTG) demonstrated that more than 50% of HR+ breast cancer recurrences occur after completion of 5 years of endocrine therapy. In this analysis the introduction of adjuvant tamoxifen for five
years demonstrated a reduction in both distant and loco-regional recurrence by up to 50%, with its effect lasting long after drug discontinuation, as well as a significant reduction in mortality of 25% compared with placebo. This led to the introduction of tamoxifen as the first standard of care adjuvant ET for HR+ breast cancer regardless of menopausal status for a duration of 5 years.7 Tumours with stronger ER expression benefit more from ET than those with low expression.6
Tamoxifen remained standard of care for all patients until the introduction of third generation AIs, which have been found to further improve disease-free survival (DFS) in post-menopausal women when used either alone or sequentially after 2-3 years of tamoxifen. They also avoid the tamoxifen-related adverse events of venous thromboembolism or endometrial carcinoma.8
In premenopausal women with high-risk HR+ early breast cancer OFS in combination with tamoxifen or an AI reduces recurrence risk compared to tamoxifen alone.9-11 Long term follow-up identified a sustained reduction in recurrence risk with exemestane/ OFS versus tamoxifen/OFS, but this has not translated to an overall survival (OS) benefit at 12 years (HR 0.93, p=0.43). However, an OS benefit was observed in a small cohort of trial patients deemed ‘high-risk’ (age younger than 35, high tumour grade 3 or tumour larger than 2cm in size, leading Pagani et al to conclude that it is important to select out the appropriate cohort of premenopausal women who benefit from OFS given the effect on quality-of-life observed with treatment intensification.12
Determining menopausal status is paramount to selecting appropriate endocrine therapy. If uncertainty arises potential consideration for treatment should include using tamoxifen treatment alone or medical/ surgical induction of menopause with GnRH agonists or oophorectomy.10
Duration of adjuvant endocrine therapy
Current standard duration of treatment for a patient with low risk, node negative HR+ disease
is five years of adjuvant therapy, with newer evidence supporting an extension of 7 to 10 years in those with higher risk disease.6, 8
The ATLAS and ATTOM trials randomised patients with early HR+ breast cancer regardless of menopausal status to either 5 or 10 years of tamoxifen treatment. Both trials demonstrated that extended therapy of 10 years reduced breast cancer recurrence and mortality compared to 5 years of treatment, by 3.7% and 2.8% respectively as per the ATLAS trial. However, an extended course of tamoxifen was associated with an increase in adverse events observed, most concerningly the risk of developing an endometrial cancer or a thromboembolic event.13, 14
Table1: Pivotal adjuvant endocrine therapy trials in early hormone positive breast cancer
Guidelines changed with the introduction of the better tolerated third generation aromatase inhibitors (AIs: anastrozole, letrozole and exemestane).15
AIs provided a further small improvement in disease-free survival after breast cancer diagnosis when compared with tamoxifen in post-menopausal women and offer an improved side-effect profile.16 Further work has assessed the impact of ovarian function suppression in pre-menopausal women which can then allow an AI to be used.
A study assessing AI and OFS versus tamoxifen and OFS in premenopausal woman with early HR+ breast cancer demonstrated a greater reduction in recurrence rate with an AI/OFS (absolute 5-year recurrence risk of 6.9% versus 10.1% with Tamoxifen/OFS) but no difference in mortality between

groups was observed(p=0.94), longer follow up is required.17
Multiple studies were designed to assess the benefit of an extended duration of AI therapy early HR+ breast cancer in the postmenopausal setting, as outlined in table 1.18-26 Clinical trials extending therapy from 7-8 years to 10 years (IDEAL and ABCSG 16) failed to show better clinical outcomes with the longer duration of treatment.22, 23 The breast cancer risk profile of study participants in those trials may be one of the reasons for negative results, such as in the ABCSG 16 trial where 65% of patients with early breast cancer were nodenegative and where 73% had T1 disease.23 On balance we consider that for the majority of patients with intermediate risk disease, 7 years appears to be the optimal
duration, and in those with highrisk features, endocrine therapy up to 10 years may be considered.
When interpreting these trials into clinical practice an individualised patient approach is required, especially amongst women with low-risk cancers in whom the possible toxicities of treatment must be weighed against minimal potential improvements in clinical outcome.
Unfortunately, adherence to adjuvant ET is often poor with rates of adherence at five years reported to be as low as 66.2% in multiple different populations. Factors such as presence of co-morbidities, depression and experience of adverse events are associated with lower adherence
Table1: Pivotal adjuvant endocrine therapy trials in early hormone positive breast cancer
Endocrine Therapy Total Duration of ET therapy
Aromatase inhibitor
Letrozole
Anastrozole 10 years Vs 5 years AI MA17R[19] 1918 4.5-6 year AIs +/or TAM Any duration
5-6 years vs 7-8 years
Vs
or
TAM: Tamoxifen, AI: Aromatase inhibitor, ANA: anastrozole, LET: letrozole, aTTom: Adjuvant Tamoxifen – To offer more?; ATLAS: Adjuvant Tamoxifen Longer Against; NSABP 42: National Surgical Adjuvant Breast and Bowel Project (NSABP 42); DATA: different durations of adjuvant anastrozole therapy; IDEAL: Investigation of the duration of extended adjuvant letrozole; ABCSG 16: Austrian Breast Cancer Study Group Trial.
TAM: Tamoxifen, AI: Aromatase inhibitor, ANA: anastrozole, LET: letrozole, aTTom: Adjuvant Tamoxifen – To offer more?; ATLAS: Adjuvant Tamoxifen Longer Against; NSABP 42: National Surgical Adjuvant Breast and Bowel Project (NSABP 42); DATA: different durations of adjuvant anastrozole therapy; IDEAL: Investigation of the duration of extended adjuvant letrozole; ABCSG 16: Austrian Breast Cancer Study Group Trial. Sig: significant; Non-sig: non-significant. DFS: disease-free survival, OS: overall survival. DRFS: distant relapse free survival. LN+: lymph node

with ET whereas use of AIs and prior chemotherapy are associated with better adherence.27-29
The most common side effects of ET are hot flushes, night sweats, vaginal dryness, decreased libido and mood disturbances which contribute to poor treatment adherence. Premenopausal patients, especially when receiving OFS, often experience more side effects of ET due to the sudden drop in oestrogen levels.
Tolerance and adherence are important issues to address in survivorship breast clinics particularly when extended adjuvant ET is being considered.
Non-pharmacological interventions such as regular exercise, a healthy diet, minimal alcohol intake, smoking cessation, weight management and cognitive behavioural therapy should be recommended as part of patients’ survivorship care and may also help to ameliorate adverse effects associated with ET.30 Proactive symptom management such as symptom assessment, education and appropriate interventions (pharmacological or behavioural), improve symptom control.31 Symptoms occurring due to oestrogen deficiency are best treated with oestrogen replacement, however this is contra-indicated in a woman with any history of HR+ breast cancer
Table 2: Adjuvant CDK4/6inhibitors in adjuvant HR+ HER2- breast cancer
as it increases the risk of breast cancer recurrence.32
Oncologists may use some medications ‘off-label’ such as anti-depressants and anti-convulsants which have demonstrated significant reductions in hot flush frequency and intensity in clinical trials[33-36]. In one randomised controlled trial venlafaxine reduced hot flushes by 61% within 4 weeks of treatment.37 Gabapentin and the acetylcholine inhibitor oxybutynin have also been shown to reduce hot flushes more than placebo in clinical trials.35, 38 Fezolinetant is the most recently approved medication for hot flush management, this neurokinin (NK) 3 receptor antagonist demonstrated successful initial and prolonged management of vasomotor symptoms with an acceptable safety profile among menopausal women without cancer however, close monitoring of liver function tests is required.39, 40 This year it has been announced that a Phase III study of the NK3 antagonist elinzanetant has met all primary endpoints demonstrating a statistically significant reduction in frequency of moderate to severe vasomotor symptoms (VMS) caused by adjuvant endocrine therapy compared to placebo in women with or at high risk of developing HR-positive breast cancer.41
Another common ET-associated symptom is vaginal dryness/ genito-urinary symptoms which can result in severe discomfort for patients. There are several non-oestrogen and oestrogencontaining vaginal tablets or lubricants which can provide significant relief to patients and have demonstrated to be safe to use in the context of a previously diagnosed HR+ breast cancer.42 Women on tamoxifen who present with abnormal gynaecological symptoms, such as vaginal bleeding or discharge, should be investigated promptly with a gynaecological review +/- ultrasound pelvis/endometrial biopsy. Tamoxifen has tumourpromoting activity in the uterus and has been associated with an increased risk of endometrial hyperplasia, polyp formation and more rarely invasive carcinoma and uterine sarcoma.43
There is approximately a 2-3-fold increase in the risk of thromboembolic events in healthy women treated with tamoxifen, factors increasing this risk are personal or family history of VTE, severe obesity, smoking and increasing age. Prophylactic anti-coagulation is not routinely recommended but can be considered in certain cases such as women with multiple VTE risk factors unable to use an AI. Following development of a VTE
on tamoxifen patients require therapeutic anticoagulation and consideration of alternative ET.44
Thromboembolic events and gynaecological malignancies are not associated with AIs. However, AIs can be associated with aromatase-associated arthralgia which presents as joint pain and swelling and is treated with analgesia and gentle exercise but in some cases drug discontinuation is required. AIs are also associated with decreasing bone mineral density; therefore, women commencing an AI should undergo a baseline DEXA scan and be treated with a bisphosphonate if required alongside calcium and vitamin D supplementation. A preexisting diagnosis of osteoporosis is not a contra-indication for AI use but appropriate treatment should be commenced.45
In younger patients’ commencing chemotherapy or ET, fertility goals should be addressed. High-risk premenopausal patients benefit from OFS for several years to improve OS and recurrence risk. Women of childbearing age must be educated that tamoxifen is a teratogenic agent and a wash out period of 3 months is recommended before attempting to conceive.
The POSITIVE trial investigated temporary interruption of adjuvant
Table 2: Adjuvant CDK4/6inhibitors in adjuvant HR+ HER2- breast cancer
Monarche 3 Pre/ postmenopausal HR+ High risk: - >= 4 axillary LN - 1-3 axillary LN and Histological Grade3 Or tumour size >5cm
Natalee 3
menopausal HR+ Stage II or III disease
Pallas 3 Pre/ postmenopausal HR+ Stage II or III
1 off
ET +/Palbociclib 125mg OD 3 weeks on/ 1 off
Sig: significant. Not sig: not significant. ET: Endocrine therapy. HR+: Hormone positive IDFS: invasive disease-free survival, OS: overall survival.
significant. Not sig: not significant. ET: Endocrine therapy. HR+: Hormone positive IDFS: invasive disease-free survival, OS: overall survival.
Stage II or III 125mg OD 3 weeks on/ 1 off
Sig: significant. Not sig: not significant. ET: Endocrine therapy. HR+: Hormone positive
IDFS: invasive disease-free survival, OS: overall survival.

ET after at least 18 months of treatment in young women (42 years or younger) with stage I-III disease to attempt pregnancy. The 3-year incidence rate of breast cancer events was 8.9% compared to 9.2% in the no interruption cohort indicating a temporary interruption of therapy for pregnancy did not demonstrate a negative short-term effect in lower-risk groups but longer follow up is required.46
Adjuvant bisphosphonates
AI use can lead to a reduction in bone mineral density and subsequent bone fractures. In post-menopausal women receiving AI treatment or premenopausal women receiving OFS, bisphosphonates are felt to not only have a role in the prevention and/or treatment of bone loss but may also improve breast cancer survival. The EBCTCG meta-analysis found statistically significant benefit for bisphosphonates in all postmenopausal patients with breast cancer for bone recurrence, fracture rates, breast cancer mortality and OS.47 These differences did not vary as a function of treatment features (bisphosphonate class, treatment schedule, and dose). As with all treatments, the balance of potential clinical benefit versus potential toxicities should be weighed carefully with patients.47-49
The most well-studied adjuvant bisphosphonate is intravenous zoledronic acid however oral agents are often prescribed to avoid the need for IV infusions.50-52
CDK4/6 inhibitors
The management of HR-positive, HER2 negative metastatic breast cancer has recently changed to incorporate CDK4/6 inhibitors (Abemaciclib, Ribociclib and Palbociclib). The combination of CDK4/6 inhibitors and ET when compared to ET alone improves both progression free survival and OS in advanced breast cancer.53, 54 More recently, their role and benefit in early-stage HR-positive, HER2 negative breast cancer has been published in both the Monarche (Abemaciclib)55 and Natalee (Ribociclib)56 studies as shown in table 2.
Monarche randomised women with high-risk early breast cancer (node positive) to endocrine therapy with or without Abemaciclib 150mg orally twice a day for 2 years followed by continued ET. At four years follow up the absolute difference in IDFS with Abemaciclib was 6.4%. A continued benefit was seen post completion of 2 years of Abemaciclib.55 At 5-years a sustained benefit of Abemaciclib versus placebo in IDFS is seen however, despite fewer deaths observed in the Abemaciclib arm (208 vs 234), overall
survival data did not reach statistical significance.57
The NATALEE trial investigated 3 years of adjuvant Ribociclib with a non-steroidal aromatase inhibitor (NSAI) compared to NSAI alone, both followed by continued ET in patients with stage II or III breast cancer. Like Monarche, a significant improvement was seen in IDFS but not overall survival with the addition of Ribociclib.
No benefit was observed with the addition of adjuvant palbociclib to standard ET as per the PALLAS trial.58
It remains to be seen whether the addition of abemaciclib or ribociclib to adjuvant ET will ultimately improve overall survival compared with standard ET alone and with the initiation of CDK4/6 inhibitors at time of metastatic relapse. CDK4/6 inhibitors are associated with significant toxicity and require relatively intensive monitoring and surveillance in dedicated clinics. Additional treatment is associated with an increase in financial, time and drug toxicity which should be borne in mind when prescribing for healthy breast cancer survivors. Abemaciclib and ribociclib are both approved by the FDA and EMA but only abemaciclib is available for public patients in Ireland for use in adjuvant high-risk HR+ breast cancer patients.
What treatments do we recommend in our practice for patients with HR+ early breast cancer?
All suitable patients should be offered adjuvant endocrine therapy with several evidence-based and reasonable options available as outlined in Figure 1.8, 25
Adjuvant anti-hormonal therapy is an effective intervention to reduce recurrence rates and improve overall survival among patients with hormone-receptor-positive early breast cancer. Almost all patients will benefit from at least 5 years of anti-hormonal therapy with additional combinations of bisphosphonates, ovarian function suppression, CDK 4/6 inhibitors and/or extended adjuvant endocrine therapy appropriate for some patient subgroups. Oncologists play an important role in explaining the rationale for adjuvant endocrine therapy to breast cancer survivors, the potential incremental benefits of additional treatments as well as their associated potential toxicities. A flexible and responsive oncology clinic for the management of patients on adjuvant endocrine therapy is best positioned to support patients and maximise adherence to treatment.
References available on request


Introduction
Multiple myeloma (MM) is an incurable haematological malignancy characterised by clonal expansion of abnormal plasma cells in the bone marrow. MM affects around 384 people in Ireland each year, and the median age of diagnosis is 65 years. A diagnosis of MM is indicated by greater than 10% plasma cells in the bone marrow, and evidence of end-organ damage (hypercalcemia, renal insufficiency, anaemia and bone lesions). Typical treatment of MM comprises a combination of several drug classes, normally three or four, including proteasome inhibitors, immunomodulatory agents, monoclonal antibodies, alkylating agents and corticosteroids. Treatment aims to get the patient into deep remission, to achieve the best chance of progression free survival, as eventually all patients will relapse.
The current standard-of-care treatment approach is to treat MM continuously to maximise treatment response and achieve deeper remission, both at diagnosis and relapse. As a result, patients require many hospital visits, may experience continuous side effects, and additionally this has financial implications. Chimeric antigen receptor (CAR) T-cell therapy has changed this treatment paradigm approach. CAR T-cell therapy is administered as a single treatment and provided a patient responds, does not require continuous maintenance therapy. CAR T-cell therapy has emerged in recent years as a promising treatment strategy for many blood cancers. CARs are genetically engineered T-cell
Written by Roisin McAvera, Senior Postdoctoral Researcher, Royal College of Surgeons in Ireland
receptors designed to target a tumour-associated antigen and promote T-cell mediated death. CAR T-cell therapy is currently FDA approved for both early and later stages of MM.
Currently available CAR T-cell therapies are manufactured specifically for each individual patient (Figure 1). The patient undergoes leukapheresis, a process where white blood cells are separated from blood in order to isolate T-cells. In a laboratory, these T-cells are genetically modified to express a CAR targeting the desired tumourassociated antigen. CARs are hybrid molecules that contain both an antigen recognition domain and T-cell activation domain. The new CAR T-cells are then stimulated to expand ex-vivo using growth factors until there are enough to proceed with infusion back into the patient. This manufacturing process can take 6 weeks, and so the patient receives bridging therapy in the meantime. Once infused, the CAR T-cells continue to expand within the patient’s bloodstream, bind to malignant
cells and orchestrate recruitment of immune cells to kill the malignant cells.
To ensure optimal efficacy, CAR-T target antigens should be highly expressed on malignant cells, with little to no expression on non-malignant cells. There are currently two CAR T-cell therapies approved for MM, ide-cel and cilta-cel, which both target B-cell maturation antigen (BCMA). BCMA is highly expressed on MM cells, and its expression remains high even at relapse and in patients with high-risk extramedullary disease. Importantly, BCMA is not expressed on haematopoietic stem cells or non-haematological cells making it an ideal target. CAR T-cell therapy targeting BCMA has improved outcome for both standard-risk and highrisk patients, however high-risk patients still relapse in a shorter amount of time. Other targets under investigation include GPRC5D and SLAMF7 which are both highly expressed on the surface of MM cells. Therapies targeting both are in early phase clinical trials and have shown promising results in the relapsed/ refractory settings.
Unfortunately, there are toxicities associated with CAR T-cell
therapy. The most common is cytokine release syndrome (CRS) which is a systemic inflammatory response where high levels of cytokines are released from immune cells into the bloodstream. This can be characterised by nausea, fever and multiple organ failure. Many patients will experience low-grade CRS which is tolerable, but those who experience high-grade CRS may need clinical intervention. Another complication associated with CAR T-cell therapy is immune effector cell-associated neurotoxicity syndrome (ICANS). This is a neurological toxicity thought to be a manifestation of CRS. Presentation can range from loss of cognitive function to seizures and cerebral oedema.
Like all therapies for MM, a huge clinical challenge is patients developing resistance. There are many factors that impact drug resistance, all ultimately resulting in selection of a subclonal population of resistant cells. For example, BCMA-directed therapy will target cells expressing BCMA, but a small proportion of malignant cells without BCMA will survive and eventually expand resulting in clonal evolution. This new clonal population of cells will not respond to the therapy leading to relapse. As a result research is continuously searching for novel CAR-T targets for potential use in later disease stages.
There are also several logistical challenges associated with CAR T-cell therapy. Firstly, the manufacturing process is lengthy and in some cases may not be successful due to factors such as low white blood cell count in the sample or effects of medication. Additionally, this therapy is significantly costly. In the UK, the cost is reported as £280,000 (around ¤337,500) per patient.
CAR T-cell therapy is a promising treatment approach for MM and will undoubtedly change the future treatment paradigm. However, patients with high-risk features still ultimately have the worst outcome, and therefore should still remain the focus of future novel therapies. There are several clinical and logistical challenges for CAR T-cell therapy, but as research continues hopefully these will be minimised.
References available on request

Written by Alessandra Allotta*, Yasmine Maati Chaibi*, Dr. Daniela Ottaviani, Dr. Jason McGrath, Dr. Damir Vareslija, and Professor Leonie Young.
Department of Surgery, Royal College of Surgeons in Ireland (RCSI)
Contributed equally*
Overview of Breast Cancer Brain Metastases (BCBMs)
Breast cancer is one of the leading causes of cancerrelated mortality among women globally. In advanced disease, brain metastases develop in approximately 10–16% of patients,1 with a higher incidence in aggressive subtypes such as HER2-positive and triple-negative breast cancers.2 Once the central nervous system (CNS) is involved, outcomes worsen significantly: median survival ranges from 4 to 26 months, depending on various clinical factors.3,4 These poor prognoses are largely attributed to the restrictive nature of the blood-brain barrier (BBB), which impedes the delivery of many anticancer agents.1 Conventional
1
chemotherapies often show limited efficacy, underscoring the pressing need for innovative therapeutic strategies that can effectively target BCBMs.
CDK12: A Key Cyclin-Dependent Kinase
Cyclin-dependent kinase 12 (CDK12) is involved in gene transcription, pre-mRNA splicing, intron polyadenylation, and translation.5 By phosphorylating the C-terminal domain of RNA polymerase II, it directs transcription elongation and splicing of genes vital for cell cycle control and DNA repair.5,6 This includes genes required for homologous recombination, a crucial DNA repair pathway. Dysregulation of CDK12 can undermine these processes, driving genomic instability linked to cancer.7

Mechanisms of CDK12 in DNA Repair
CDK12 supports DNA repair by phosphorylating RNA polymerase II, thereby sustaining the expression of genes crucial for homologous recombination and related pathways.7 Loss or inhibition of CDK12 can compromise these repair mechanisms and spark genomic instability—an event that may accelerate tumour progression but heighten sensitivity to certain treatments. Research indicates that inhibiting CDK12 can sensitize cells to PARP inhibitors, revealing a possible therapeutic synergy.7 Further work shows that selective CDK12 inhibition disrupts transcription of key HR genes like BRCA1 and FANCD2, creating synthetic lethality when paired with PARP inhibitors.8 This underscores the potential of targeting CDK12 to capitalize on DNA repair vulnerabilities in cancer cells.

Evidence Linking CDK12 to Breast
CDK12 plays a critical role in breast cancer, with effects varying by subtype. In HER2-positive disease, it often co-amplifies with HER2, contributing to tumour growth; in triple-negative breast cancer, it appears to act as a tumour suppressor.9 It also promotes stem cell-like properties by activating WNT and ErbB-PI3K signalling.10 CDK12 rearrangements appear in 3.5% of breast cancer brain metastases, compared with 0.86% in primary tumours, suggesting a link to metastatic progression.11
Rationale for Targeting CDK12 in BCBMs
Many breast tumours in the brain depend on backup DNA repair pathways, including those controlled by CDK12.7 Blocking CDK12 taps into this weakness, making these cells more responsive to DNA-damaging treatments like PARP inhibitors and certain chemotherapy drugs.8,12 Research also shows that reducing CDK12 activity can improve how HER2positive breast cancers respond to HER2-targeted therapies by restraining the PI3K/AKT pathway.13 There is further evidence that CDK12 inhibition may increase immunogenic cell death, which could enhance immunotherapy effectiveness by raising neoantigen levels and activating immune responses.14 Collectively, these
findings suggest that targeting CDK12 could be a promising approach to managing brain metastases from breast cancer.
Therapeutic Approaches and Drug Development
Research into small-molecule CDK12 inhibitors has expanded rapidly, producing agents with different ways of targeting CDK12. Dinaciclib, which broadly inhibits multiple CDKs, also impacts CDK12 and shows promise in combination with DNAdamaging treatments or PARP inhibitors.12 THZ531 selectively and covalently blocks CDK12/ CDK13, hindering transcription of DNA repair genes.15 SR-4835 is a selective ATP-competitive dual inhibitor of these kinases and has demonstrated synergy with DNA-damaging drugs and PARP inhibitors, particularly in triple-negative breast cancer.8 HQ461 acts as a molecular glue by uniting CDK12 and DDB1, leading to cyclin K degradation and weakening DNA repair (16). Finally, PROTAC degraders PP-C8 and 7f clear the CDK12–cyclin K complex, interrupting transcription and DNA repair pathways.17,18 These approaches—whether enzymatic blockade or induced protein degradation—can heighten tumour responsiveness to existing treatments, suggesting new possibilities for managing breast cancer brain metastases and other difficult-to-treat malignancies.
(A) Treatment with PARP inhibitors (PARPi) or DNA-damaging agents (DDAs) aims to trigger tumour cell death by causing DNA damage but often faces resistance. Adding CDK12 inhibitors (CDK12i) increases genomic instability, making tumour cells more susceptible to PARPi or DDAs and enhancing treatment success. (B) HER2-targeted tyrosine kinase inhibitors (TKIs) block the ErbBPI3K pathway to induce tumour cell death but also encounter resistance. Combining CDK12i with TKIs further suppresses PI3K/ AKT signalling, improving tumour sensitivity to TKIs and offering a stronger therapeutic approach.
Preclinical and Clinical Trial Evidence
Preclinical work on CDK12 inhibition points to encouraging outcomes in breast cancer. Laboratory studies show that blocking CDK12 makes triplenegative and HER2-positive breast tumours more responsive to therapies like PARP inhibitors and HER2-tyrosine kinase inhibitors by disrupting DNA repair and survival pathways.8,12,13 In animal models, high levels of CDK12 accelerate tumour development and spread, underlining its part in tumour progression.19 Although these results are promising, patient data are still limited. A new Phase 1 clinical trial (NCT06600789) was launched in September 2024 to assess
CT7439, a CDK12/13 inhibitor and cyclin-K degrader, marking a key step in clinical development.
Challenges and Limitations
Despite the promise of CDK12 inhibitors, several hurdles remain. Specificity is essential to minimise off-target toxicity in healthy tissues. Predictive markers, such as homologous recombination deficiency signatures, are needed to pinpoint which patients will benefit most. Tumours may also adapt and become resistant to CDK12 inhibition, so combination strategies could help maintain effectiveness. Moreover, breast cancer’s diverse genetics and behaviour require treatments tailored to each patient’s tumour profile.
Directions
Personalised medicine, through detailed molecular profiling, may identify patients who stand to gain the most from CDK12-targeted therapies.20 Combining CDK12 inhibitors with immunotherapy or PARP inhibitors is another promising area, as the DNA damage caused by CDK12 blockade may heighten responses to immune checkpoint drugs.14 Ongoing translational research and well-planned clinical trials will be essential to validate markers and refine treatment strategies, improving the outlook for individuals with breast cancer brain metastases.
References available on request



Written by Ms Katie E Johnston RD, Research Dietitian (Oncology), Cancer Research @UCC, University College Cork and Dr Samantha J Cushen PhD RD, Lecturer in Human Nutrition and Dietetics, School of Food and Nutritional Sciences, University College Cork


Whilst the number of cancer survivors are increasing, services are failing to keep up with their growing needs and long-term impact of cancer treatment on body composition, psychological health and emotional wellbeing. One cohort of particular concern are early-stage hormone-receptor positive breast cancer survivors who are prescribed an endocrine therapy up to 10 years following the completion of systemic anticancer treatment. Their unmet needs have been well documented in the literature, yet our healthcare pathways continue to fail them. They often lack adequate symptom and nutritional management, describing this part of their cancer journey like “being pushed out to sea on a boat with no oars”.1
We must ask ourselves – why does the supportive care stop if the treatment continues?
The National Cancer Registry in Ireland reported that 31.8% of invasive cancers diagnosed in 2020-2022 were breast, with breast cancer survivors making up 22% (n=49,240) of the cancer survivor population. Hormone receptor positive (HR+) /human epidermal growth factor receptor 2 (HER2-) breast cancer accounts for over 70% of all breast cancer cases diagnosed.2 The European
Society of Medical Oncology (ESMO) recommend the use of an adjuvant endocrine therapy in all HR+ breast cancers for 5 years, increasing up to 7-10 years for higher stage cancers.2 The use of an endocrine therapy can reduce the risk of breast cancer recurrence by 30-50% after 5 years of therapy and improve disease free survival.2
However, endocrine therapy isn’t without its side effects, including fatigue, body composition alterations, joint pain and insomnia. The ESMO Clinical Practice Guideline reports that side effects of adjuvant endocrine therapy affect women of all ages and should be addressed to improve quality of life and adherence.
Qualitative data from HR+ breast cancer survivors reports a desire for “transparency about weight gain” as a side effect of their cancer treatment.3 “Avoid being overweight” as noted in the ESMO guidelines is not an effective recommendation for the complexities of obesity and weight gain in this cohort, nor is it particularly helpful.
As healthcare professionals, we need to set expectations around side effects of endocrine therapies, including changes to body composition, treating them the same way we would a chemotherapy agent or radiotherapy. The conversation,
particularly around body composition alterations, should happen at the beginning of endocrine therapy treatment, imploring targeted dietetic interventions to manage side effects, support long-term health and reduce recurrence risk.
The ESMO guidelines recommends that these issues have a significant influence on quality of life and deserve close multidisciplinary team (MDT) follow up. In the Irish sphere, recent research reported that whilst 60.9% of breast cancer survivors surveyed desired dietetic support, they were the least likely to receive it (17%).4
Questions to ask ourselves:
• Do we need to reframe how we think about endocrine therapy?
Many patients are told they have completed curative treatment for their cancer but still experience endocrine therapy side effects that can be more debilitating than the chemotherapy, radiotherapy or surgery itself. Given this, how should we reconsider the role and impact of endocrine therapy in post-treatment care?
• Who should these patients be referred to?
Traditionally, dietetic care during cancer treatment has focused on malnutrition however, in light of the epidemic of nutrition misinformation and black-market weight loss drugs, registered oncology dietitians should be consulted as part of the MDT to provide safe, evidence-based weight management strategies.
• Where should they be referred?
Should MDT care, including dietetic care, be provided at medical oncology outpatient clinics? In the community, where these patients are usually referred, weight management services are not seen as the priority and patients are often put on a waiting list. Unfortunately, these women are falling through the gaps in our cancer care services.
Nutritional Considerations in HR+ Breast Cancer Survivors and the Role of the Oncology Dietitian
The role of an oncology dietitian, particularly in the survivorship phase, is complex and multifaceted (Fig 1). Many cancer survivors are balancing recovering from treatment to getting back to pre-treatment life. Furthermore, many HR+ breast cancer survivors are trying to manage the side effects of endocrine therapy, which directly impacts nutritional intake and body composition.
The nutritional considerations in HR+ breast cancer survivors extend beyond just “weight”, encompassing heart health, bone mineral density, sarcopenic obesity, gut function, disordered eating behaviours and dietary supplement use, among others. These nutritional considerations, and the ability for the patient to manage them, go hand-in-hand with endocrine therapy related effects, such as fatigue, insomnia, and body composition changes. In Ireland, concerningly, 40.6% of cancer survivors surveyed reported conducting self-directed research on nutrition, diet and cancer.4
Body Composition Changes and Weight Gain Concern
Muscle mass is an important prognostic factor in cancer that has been well documented in the literature,5 however it is not routinely assessed in HR+ breast cancer survivors due to a myriad of factors. A lack of standardised protocols, limited access to specialised equipment such as bioelectrical impedance analysis or DEXA scans, and limited trained personnel are all barriers to muscle mass assessment in outpatient clinics.6 Whilst assessing muscle mass may not be seen as a high priority in outpatient clinics, muscle mass is directly related to adjuvant endocrine therapy outcomes. Muscle mass and toxicity-related adjuvant endocrine therapy discontinuation have been


independently associated, with low muscle mass increasing toxicity related discontinuation (OR 2.18, p = 0.036).7 Furthermore, toxicity related discontinuation was associated with worse ipsilateral breast tumour recurrence and diseasefree survival (HR 9.47, p = 0.002; HR 4.53, p = 0.001).7 In breast cancer, a further consideration is sarcopenic obesity, higher levels of adiposity with the lowest levels of muscularity. It is independently associated with higher mortality and higher rate of complications in systemic and surgical cancer treatment across multiple cancer types.7
The Role of the Oncology Dietitian Clinicians often prioritise immediate concerns in cancer survivors at outpatient clinics, e.g. disease recurrence and treatment side effects, leaving muscle mass assessment underemphasised. The importance of muscle mass as a prognostic factor may not be fully recognised or integrated into routine care. Oncology dietitians, who have upskilled in body composition, are in the unique position to assess, prevent
and manage those at risk of low muscle mass, and sarcopenia, (the accelerated loss of skeletal muscle mass and function commonly, but not exclusively, associated with advancing age8). Personalised nutritional counselling is vital to managing low muscle mass and sarcopenia risk, by ensuring optimal protein intake throughout the day. For individuals with a cancer diagnosis, guidelines for protein intake recommend 1.2g –2.0g protein per kilogram of body weight per day (kgBW/d),9 with the higher end of this range being significantly above the average intake for most individuals.10
Research published in 2020 reports that protein intake in the Irish population declines with increasing age, and intake is skewed across the day.11 This decline may increase the risk of sarcopenia, especially in older adults with cancer.
Cancer disrupts the homeostatic state of muscle protein turnover, accounting for increased nutritional recommendations for protein. Amino acids, the dietary anabolic drivers of muscle mass accretion, vary in quality and do not equally promote anabolism.
Animal proteins provide better anabolic stimuli compared to plant protein foods and a combination of animal proteins, contributing to >65% of total protein intake, and plant proteins is recommended for optimal muscle health.9
Assessing muscle mass is not routinely captured in medical oncology outpatient clinics, despite being cited as one of most common nutritional problems – and likely most impactful on prognosis – in the oncology population.5 One of the gold standard body composition measurements, computed tomography, is expensive, inaccessible and exposes patients to unnecessary radiation. In recent years, novel techniques have been explored, such as bioelectrical impedance analysis or ultrasound, as viable alternatives. Routinely monitoring muscle mass allows for a pro-active approach of timely dietetic intervention instead of a reactive approach, which is the current standard. This is particularly important in HR+ breast cancer survivors, who typically look “well-nourished” and may not be assessed for low muscle mass or sarcopenia risk.
Furthermore, dietetic intervention to improve muscle mass and protein intake to encourage anabolism may prevent incidences of toxicity-related adjuvant endocrine therapy discontinuation.7
Adipose Tissue Mass and Weight Gain
Weight gain following breast cancer treatment is reported in up to 90% of patients and is associated with disease-free survival outcomes in breast cancers.12-14 Weight gain can be attributed to chemotherapy, endocrine therapy and menopausal status. Research has shown that 52% of breast cancer survivors receiving adjuvant endocrine therapy experienced a clinically significant mean weight gain after 60 months of 3.5kg (±10.4kg) in premenopausal women and 0.6kg (±6.5kg) in post-menopausal women.12 Evidence has suggested that cancer survivors living with obesity increase their total risk for mortality by 17% for every 5kg/m2 increment before their cancer diagnosis, for breast cancer specific mortality this increases to 18%.15 Breast cancer survivors

with a high adipose tissue mass are at risk for recurrence, and furthermore at increased risk of cardiovascular incidence, including those with a healthy body mass index (BMI).16-17
The Role of the Oncology Dietitian
We need to begin to move away from the simplistic approach of “eat less, move more”, acknowledging that the neurobiology of appetite, body weight and energy regulation is mediated by a combination of hormonal signals from the gut, adipose tissue and other organs,18 which in HR+ breast cancer, is likely affected further by adjuvant endocrine therapies.
The Adapted Clinical Practice Guidelines for Ireland defines obesity as a complex chronic disease characterized by excess or dysfunctional adiposity that impairs health.18 As obesity is chronic in nature, the treatment plan must be long-term, following a comprehensive medical, physical, functional, psycho-social and behavioural assessment.18 BMI alone is not an accurate stand-alone tool for identifying adiposity related complications in breast cancer survivors, however, this is the anthropometric measure routinely used in medical oncology outpatient clinics. Breast cancer survivors experiencing excess adiposity often experience substantial bias and stigmas and this dominant narrative fuels assumptions about personal irresponsibility and lack of willpower.19
Weight Gain Concern: How to Address It
Sensitive and respectful conversations around “weight management” need to be considered. Literature has reported disordered eating behaviours are prevalent in both young female cancer survivors and women of menopausal ages.20-23
A fixation on weight and weight loss following breast cancer treatment or during endocrine therapy, without adequate dietetic support, may lead to risk of orthorexia nervosa (ON), a disordered eating behaviour characterized by an exaggerated fixation on health-conscious eating behaviours.20 In ON, behaviours seen outside consuming the “purest” of food can lead an individual to experience severe emotional distress due to the rigid pressures.20 This behaviour
may lead to the decreased consumption of important macro and micronutrients.
Changing the focus from “weight loss” to “health gain” is key when providing nutritional support and counselling to this vulnerable cohort of patients, highlighting important changes to support their long-term health (e.g. cardiovascular risk, bone health).
Taking the time to hold space in consultations to address weight gain concern and actively listening to their lived experience will create a safe environment for these patients. Weight gain can be a result of a myriad of factors, including endocrine therapy related side effects such as fatigue, insomnia and joint pain. Working collaboratively with registered dietitians with experience in weight management and oncology can ensure a safe, evidence-based treatment plan can be put in place for these patients.
Questions to ask ourselves:
• How are we addressing weight gain in this cohort of patients?
Are you holding space in your consultations for this conversation or is it a throw away comment such as “watch your weight” or “expect weight gain”.
• Are you weight bias?
Whilst it may not be an intentional practice, completing a selfassessment tool to address weight bias is vital as a healthcare professional providing care to this particularly vulnerable cohort.
Use the Implicit Association Test at https://implicit.harvard.edu/ implicit/takeatest.html selecting the Weight IAT test.
• Are we referring patients to specialists in the area, given the complex nature of weight management in this cohort?
Patients will seek out information from somewhere, so what are we doing to provide them with safe, evidence-based information? Are dietitians being included in the MDT? Are medics and nurses consulting dietitians about patient cases, even if the service isn’t available?
Bone Mineral Density
Females undergoing cancer treatment can experience accelerated bone loss, within 6 months of treatment, resulting in a substantial 21% decrease in bone density compared to agematched menstruating females.24,25
Furthermore, postmenopausal breast cancer survivors prescribed an aromatase inhibitor and gonadotropin-releasing hormone (GnRH) agonist experience bone loss at a yearly rate 7.7% higher than the general population.26
The Role of the Oncology Dietitian
As per the American Society of Clinical Oncology (ASCO) and ESMO guidelines, Calcium and Vitamin D supplementation of 1000mg and 800IU per day is recommended to support bone mineral density.2,27 Anecdotally, breast cancer survivors find the taste and texture of prescribed combined calcium and Vitamin D supplements “chalky” and often turn to over the counter supplements in pharmacies or health food stores. Ensuring that patients have access to acceptable alternatives is crucial for supporting bone health. However, it’s important to note that supplements should complement/ supplement, not substitute prescribed treatments. Based on our research in survivorship clinics with this cohort, the rising trends of plant-based dairy alternatives and organic food is a cause for concern, particularly when the nutritional value of these products is misunderstood. In Ireland, approximately 11% of adults aged 19-64 years and 7% of >65 years reported consuming non-dairy milk alternatives.10 If diets are not carefully planned, essential nutrients, like protein, iodine and calcium, may be unintentionally excluded. For individuals who choose these alternatives, working with a registered dietitian to carefully assess and plan their diet can help ensure they meet all their macro- and micronutrient requirements.
Supplement Use
Supplement use is highly prevalent in breast cancer survivors.28,29 The global “menopause-specific” supplement market is growing rapidly, projected to grow from USD 17.77 billion in 2024 to USD 25.89 billion by 2031.30
Harrigan et al.,28 reported 83% of breast cancer survivors surveyed (n=475) used a dietary supplement, with 108 different supplements reported. Concerningly, there were 36 supplements reported that had a potential adverse interaction with tamoxifen or an aromatase inhibitor.28 Of n=353 women prescribed an endocrine therapy, 38% were taking a dietary supplement with a potential risk of interaction, including black
cohosh, turmeric, ginseng, milk thistle and slippery elm bark.28
The Heads of Food Safety Agency In Europe established a Working Group on Food Supplements to create a common approach on to the management and assessment of ingredients or nutrients that could pose a risk to public health when used in food supplements.31 The group evaluated over 117 dietary supplements and identified 13 as potential health risks – these included maca root, ashwagandha, tea tree oil, curcumin, tryptophan, black cohosh and melatonin.31 These substances could face restrictions, bans, or further evaluation by the European Food Safety Authority (EFSA), with final decisions expected in the coming years. The goal is to enhance consumer protection and create uniform supplement regulations across the EU.
Dietary supplements are only required in those with a diagnosed nutritional deficiency via a blood test or those following a restricted diet, such as veganism. In Ireland, supplements are regulated as food products rather than medicines, meaning they fall under food safety laws and regulations. Regulations for dietary supplements are less strict than prescription medications. Those who manufacture the supplement are responsible for ensuring that the products being placed on the market are safe and compliant with the legislation. While certain nutrients may offer beneficial effects when taken as supplements (e.g. Vitamin D), it is important to recognise that many nutrients can have adverse effects if consumed in excessive doses. Supplement companies often exceed the Nutrient Reference Value (NRV) in their products to account for potential nutrient degradation over time. Since food law requires the nutritional composition on labels to reflect the average value of a nutrient over the full shelf life of the supplement, manufacturers add extra nutrients at the outset to ensure that the product meets its labelled content even after months of storage. Currently, no NRVs have been set by European Law for herbal/ botanical ingredients. There are no maximum safe levels for these ingredients in Ireland.
The risk of micro-influencers and celebrities producing their own
dietary supplements has risen exponentially in the last number of years.32
Ensuring that survivors are meeting macro and micronutrient requirements through dietary analysis and education and having opening conversations about supplement use for symptom burden, may reduce the potential health risks that using dietary supplements may cause.
Survivors may often be reluctant to discuss supplement use with their medical oncology team.33,34 By having and open and honest conversation with survivors about supplement use and what they hope to achieve with it, we can potentially mitigate harmful outcomes, like herb/nutrientdrug interactions.33 Furthermore, actively consulting with the wider multidisciplinary team, including pharmacy and dietitians, will allow for a more thorough evaluation of the supplement and provide the opportunity for suggestive safer alternatives.
In Ireland, a 2019 report by IrSPEN highlighted that the ratio of dietitians to patients is one dietitian per 4,500 oncology patients.35 A recent survey conducted by the Irish Nutrition and Dietetic Institute reported 45.5 whole time equivalent (WTEs) dietitians working across 8 cancer centres and CHI Crumlin, an increase of 7.5 WTE over the last four years.36 In 2020 – 2022, there were 24,207 “life-changing” invasive cancers diagnosed annually, requiring extensive treatment37 meaning the ratio of oncology dietitians to newly diagnosed oncology patients, per year, is 1 to 532. This number does not account for already diagnosed patients, and we can only suspect from the data in 2019, that it is higher. There are 3 WTE dietitians working across non-cancer centres, allocated to Dublin areas.36 Furthermore, the survey reports that 70% of dietitians working in oncology are based in the Dublin areas, and the majority

of dietitians are not dedicated to oncology, with a mixed caseload.36 Currently, 87.5% of the cancer centres include dietitians as a core member of the MDT, whilst in the non-cancer centre providers, approximately only one third participate in MDTs.36 Whilst this data is promising, there are currently no dietetic services for the post-treatment phase – why does the supportive care stop?
What Could a National Dietetic Survivorship Service Look Like?
The logical next step for improving care for the post-acute treatment phase is to develop a national dietetic survivorship service. Whilst a referral to a specialist oncology dietitian for a 1:1 appointment may not be necessary for every individual patient, providing evidence-based nutrition information to support longterm health is. A tiered national service could provide the different modalities that cancer survivors, specifically for HR+ breast cancer survivors, may require at different stages of their journey (Fig. 2).
Dietetic interventions for cancer survivors are highly accepted34,38,39 and feasible in a range of modalities.40-50
Telephone consultations have shown to be feasible and effective in oncology weight management research.40,42,44,48,50 The use of virtual consultations has the potential to enhance the reach of dietitians to rural areas, reduce the burden of hospital appointments and increase engagement.
Peer to peer support is considered an important strategy to cancer survivors; and the use of group education enables a supportive environment, whilst capturing more service users and minimizing cost.42 A scoping review analysed n=37 group nutrition education programs and highlighted that group education addressed unmet needs and provided practical and social support.43 Furthermore, combining group nutrition education with other supportive care interventions (e.g. physiotherapy) can improve quality of life and body weight compared to usual care (p<0.01).46


In the Irish setting, cancer survivors surveyed had a first preference for live, online nutrition talks (26.8%) and all recognised a dietitian as the primary source of information.38 The use of print media or online resources provides survivors valued autonomy for dietary information to be read, watched and understood at their own pace.39
Providing survivors with evidencebased information, specific to their diagnosis and treatment plan through targeted resources will work to combat to the epidemic of nutrition misinformation. Sullivan et al.,4 reported that in 1073 Irish cancer survivors, 56% reported feeling “confused” by nutrition information online.
The development of a tiered national dietetic survivorship service, ranging for pre-recorded webinars, to group education, to personalized nutritional counselling will ensure that every cancer survivor has access to evidencebased nutrition information.
It has been well-documented that cancer survivors have the desire for control over their health1,34,52 and if we don’t bridge the knowledge gap, they will bridge it themselves, with potentially harmful information.
Dietitians play a crucial role in the supportive care and management of HR+ breast cancer survivors receiving adjuvant endocrine therapy, providing highly specialised and multifaceted
interventions. Research is promising, highlighting the positive impact of dietetic interventions in this cohort.
In the effort to bridge this knowledge and service gap, we, the authors, have developed a five-part webinar series titled “Menopause, Diet and Cancer” in collaboration with the Irish Cancer Society, as a free patient resource for this cohort. The webinar series can be accessed on the Irish Cancer Society’s YouTube page.53
Engaging with service users, caregivers, allied health professionals and academic partners is crucial to ensuring a patient-centred survivorship service that works to be both far reaching and effective in nature. Whilst national rates of disease-
free survival are increasing, we need to ensure our survivors’ longterm health is supported.
With the cancer care sphere advancing, and survivorship services being developed across the country – we need to ask ourselves why does the supportive care for HR+ breast cancer survivors stop, when the treatment, and its side effects, continue?
References available on request
Acknowledgements:
Ms Jennifer Feighan, CEO, Irish Nutrition and Dietetic Institute
Dr Erin Stella Sullivan, Lecturer in Nutritional Sciences, Kings College London

Ms Lauren V O’Connell has been announced by the Royal College of Surgeons in Ireland (RCSI) as the recipient of the 2025 PROGRESS Women in Surgery Fellowship.
This prestigious bursary, established to promote female participation in surgical training at Fellowship level is awarded by RCSI.
Ms Lauren V O’Connell will undertake a Fellowship in advanced colorectal cancer at the Peter MacCallum Cancer Centre in Melbourne, Australia allowing her to gain international exposure in colorectal cancer surgery and
further develop her expertise for the benefit of patients.
Currently a Specialist Registrar in General and Colorectal Surgery at the Mater Misericordiae University Hospital, Ms O’Connell graduated with honours from University College Dublin in 2014. She achieved Fellowship of the Royal College of Surgeons in Ireland (FRCSI) in 2024, receiving the Professor W.A.L. McGowan Medal as the top-performing candidate in Section 2 FRCS across all RCSI surgical subspecialties.
Committed to surgical education, she has also been awarded
Ms Lauren V O’Connell, RCSI 2025 PROGRESS Women in Surgery Fellowship recipient
the Colm Galvey Educator Award (2019) for excellence in undergraduate teaching and has contributed to RCSI’s MRCS Prep Course and surgical skills workshops for trainees.
Ms O’Connell has over 35 peerreviewed publications in leading journals, has authored book chapters and has presented at major international conferences. Her research focuses on earlyonset colorectal cancer, functional outcomes post-resection, surgical education and minimally invasive techniques.
On receipt of the award, Ms O’Connell commented: “I am delighted to be the 2025 PROGRESS Women in Surgery Fellowship recipient and would like to thank RCSI for their support. This initiative will allow me to engage fully with the considerable opportunities the fellowship has to offer, developing my skills in pelvic, robotic and cytoreductive surgery for advanced colorectal cancers.
The PROGRESS Fellowship was introduced to help address the barriers to female progression in surgery and to inspire exceptional female surgical trainees, fostering a more inclusive future in the field.
Now in its sixth year, the fellowship nurtures and develops the expertise and skill base of Irish female surgeons, giving them access to international experience in their chosen fields, acquire additional surgical skills, access new technologies and contribute to the advancement of surgical science and practice on the island of Ireland.
The Fellowship was presented by RCSI President Professor Deborah McNamara as part of RCSI’s Charter Meeting.
RCSI President, Professor Deborah McNamara commented: “My warmest congratulations to Ms Lauren V O’Connell. The PROGRESS Women in Surgery Fellowship is a cornerstone of our commitment to advancing equity in surgery. Each year, we have the privilege of recognising and supporting remarkable women who embody the values of innovation, excellence, and leadership. I am confident that Ms O’Connell will continue to inspire and lead in her career, contributing to the advancement of the surgical field.”
Previous PROGRESS Fellowships recipients are Ms Ailín Rogers (2020), Ms Helen Mohan (2021), Ms Christina Fleming (2022), Ms Evelyn Murphy (2023) and Ms Ola Ahmed (2024). For more information about the PROGRESS Women in Surgery Fellowship, visit the RCSI website.

Written by Olivia Iarmak
https://orcid.org/0009-0000-5458-2489 - Data Labeling Analyst at Meta, linguist, author - and Tatiana Iarmak https://orcid.org/0000-0001-5371-2958 - Epidemiologist, author, speaker, consultant. Former lecturer at Kharkiv Regional Medical Professional College.
Aim of the study: This article explores the applications of AI in cancer management, focusing on Ireland, while drawing insights from global advancements. We will also examine the complexity of cancer, relevant statistics, and the application of AI in oncology.
Background
Cancer remains one of the most significant health challenges of our time. This life-altering disease affects millions of lives and families globally. According to CA: A Cancer Journal for Clinicians (2024), nearly 1 in 5 men or women will develop cancer in their lifetime. Around 1 in 9 men and 1 in 12 women will die from it, contributing to approximately 10 million deaths worldwide annually and leaving countless families to cope with the loss. Cancer has no age. Pediatric oncology, or childhood cancer, is a critical and delicate area of focus. While cancer is typically associated with adults, children are more vulnerable and require specialised care and treatment approaches, due to their unique developmental needs.
In the US, the National Cancer Institute states that 1 in 2 men and 1 in 3 women will develop cancer in their lifetime. The American Cancer Society’s (2025) Cancer Facts & Figures forecasts over 2 million new cancer diagnoses and 618,000 deaths in 2025.
While Ireland has a relatively lower cancer incidence rate, the Irish Cancer Society reveals that more than 44,000 people are diagnosed, and 9,800 die from it each year. Skin, prostate, breast, lung, and colorectal cancers are the most common. By 2045, the National Cancer Registry of Ireland (2019) predicts that the number of new cases could double. This is concerning, as it shows a growing trend. On a global scale, survival rates vary by individual. However, Irish Cancer Society estimates that 6 in 10 cancer patients in Ireland now survive for 5 years or more.
To address this, substantial investments are being made globally and in Ireland to combat this multifaceted disease. The study published in JAMA Oncology (2023) estimates that the global economic burden of cancer will be
$25.2 trillion from 2020 to 2050. In 2025, the Irish government allocated ¤41 million to cancer services as part of the National Cancer Strategy 2017-2026. However, the financial burden extends beyond the public investment, as individuals also face heavy costs. The Irish Cancer Society (2019) reports that cancer patients incur significant additional expenses, averaging between ¤756 and up to ¤1,000 monthly.
In recent decades, artificial intelligence (AI) has begun transforming oncology. AI involves using algorithms to simulate human intelligence and perform tasks such as data analysis, pattern recognition, and decisionmaking. In oncology, AI uses computational algorithms and data-driven tools to improve cancer detection, diagnosis, and personalised treatment strategies. This emerging field, known as computational oncology, combines AI with cancer care to offer new solutions that aim to improve survival rates. By shifting away from trial-and-error methods, AI allows precise, science-backed approaches and helps reduce diagnostic errors. In addition to improving patient outcomes, AI has the potential to reduce healthcare costs. However, while its cost-effectiveness is promising, continued investments in development and access to high-quality data are needed to fully realise its potential.
The human body contains approximately 30 trillion cells that grow, divide, and die, with new cells replacing them in a controlled cycle. When this process is disrupted, damaged cells multiply uncontrollably, causing mutations in DNA and altering cellular metabolism. This disruption leads to the Warburg effect, where cells rely


on glycolysis for energy, even with oxygen present. In type 2 diabetes and obesity, insulin-like growth factors (IGFs), elevated glucose levels, and insulin resistance promote glycolysis, accelerating mutations and cellular disruptions. This creates an environment that supports abnormal cell growth and leads to tumor formation, increasing the risk of cancer. Tumors (lumps of tissue) can be benign or malignant, depending on how they grow and spread.
A benign tumor is a non-cancerous lesion that remains localised and does not invade nearby tissues. Although, it may become larger and presses on nearby organs, causing symptoms, it does not usually turn into cancer. Unlike malignant tumors, benign tumors do not follow the Stage I-IV classification system. Instead, they are usually classified based on size, location, and potential effects on the body. Examples include lipomas (fat tissue growths) and uterine fibroids.
In contrast, a malignant tumor is cancerous from the start and has the ability to spread, or metastasise, to distant parts of the body. Cancer often begins silently, without symptoms, and
progresses through four stages. In Stage I, it is small and localised. In Stage II, it may grow or spread to nearby lymph nodes. In Stage III, it invades deeper tissues. By Stage IV, it spreads to distant organs. Malignant tumors are assessed using the TNM system for staging and grading to determine prognosis and guide treatment decisions.
Cancer originates from normal or progenitor cells that acquire genetic mutations. When mutations occur in stem cells, they can give rise to cancer stem cells (CSCs), which possess the ability to self-renew and create new cancer cells. These cells are often unpredictable and more resistant to traditional cancer treatments. CSCs can remain dormant during treatment and cause the tumor to regrow after therapy ends, contributing to relapses. They are believed to play a crucial role in tumor initiation, progression, and recurrence. There are more than 100 types of cancer, usually named after the organs or tissues where they form, such as brain cancer or pancreatic cancer. Cancer can originate in any organ or tissue without exception. The cancer types can be further categorised based on


the specific tissue or cell type involved, like carcinomas (epithelial cells), sarcomas (connective tissues), leukemias (bloodforming tissues), lymphomas (lymphatic system), and others. Viruses cause about 20% of tumors worldwide (American Society for Microbiology, 2019). These include Epstein-Barr virus (Burkitt’s lymphoma), HPV (anogenital cancer), HBV and HCV (liver cancer), Helicobacter pylori (stomach cancer), HHV-8 (Kaposi’s sarcoma), MCPyV (Merkel’s sarcoma), HIV-1 (Kaposi’s sarcoma, cervical cancer), and CMV (glioblastoma). Helminths like Schistosoma (bladder cancer) and Clonorchis (gallbladder cancer) also play a role.
Cancer and cardiovascular diseases (CVDs) share common risk factors, including stress, smoking, obesity, physical inactivity, chronic infections, and an unhealthy diet. Additionally, certain cancer treatments can harm heart health. Chemotherapy and radiation therapy, for example, may lead to cardiomyopathy, arrhythmias, and coronary artery disease (Mayo Clinic Proceedings, 2020). Consequently, cancer survivors face a higher risk of CVDs, with cardiovascular mortality sometimes surpassing cancer-related deaths (BMJ Oncology Journal, 2023).
The exact cause of cancer is not fully understood. However, several factors contribute to i ts development:
• Genetics plays an important role, especially inherited mutations in genes like BRCA1 and BRCA2. These genes normally repair damaged DNA, but when mutated, they increase the risk of cancers, such as
Ireland’s and Global Efforts in AI Oncology
breast and ovarian cancer. The effects of these mutations typically do not show up until until adulthood, often appearing in the 30s or 40s.
• Random mutations, often influenced by poor lifestyle choices (smoking, unhealthy diet) or environmental exposures (excessive UV exposure, radiation), lead to changes in genes like TP53, which help control cell division.
• Familial factors, a mix of genetic and environmental influences, also increase cancer risk. For example, Familial Adenomatous Polyposis (FAP) is caused by a mutation in the APC gene, which regulates cell growth.
As a complex disease, cancer exists in multiple forms, which makes diagnosis and treatment challenging. In many cases, a cancer diagnosis means an uncertain future, with some types being treatable while others are less so. Unfortunately, a significant percentage of cancer cases are diagnosed at advanced stages (III or IV), where treatment options are limited and survival rates are lower. Cancer treatments are still expensive and sometimes ineffective. Moreover, one-size-fits-all approach to treatment has proven to be insufficient. Cancer requires a more personalised strategy based on the unique characteristics of each case. Precision medicine can complement or replace the traditional approach. By integrating AI into early detection, diagnostics, and treatment planning, we can enhance our understanding of cancer’s complexity and improve outcomes through more precise and timely interventions.
Ireland, along with leading institutions in the United States, Australia, Asia, and Europe, is actively working to implement AI-powered tools to transform oncology. Irish hospitals and research institutions are making pioneering efforts in the integration of AI into oncology, promising a long-lasting impact both locally and globally. University College Dublin (UCD) is at the forefront of multiple initiatives aimed at enhancing early detection, diagnostics, and treatment through machine learning and AI algorithms. Notably, UCD’s Systems Biology research centre coordinates Precision Oncology Ireland (POI), a consortium of Irish universities, cancer research charities, and international companies. Additionally, All-Island Cancer Research Institute (AICRI) brings together ten academic institutions (UCD, TCD, UL, RCSI, TU Dublin, DCU, QUB, UU, NUIG, and UCC) and maintains a transatlantic partnership with Roswell Park Comprehensive Cancer Center in Buffalo, New York. While AI adoption in Ireland is still in its early stages, research partnerships, clinical trials, and collaborations with global companies are accelerating progress in both urban and rural settings. Similarly, top institutions worldwide are making groundbreaking strides in AI-driven cancer management. In the U.S., the Mayo Clinic has created an AI model capable of detecting pancreatic cancer in CT scans as much as 475 days before a clinical diagnosis. It also uses AI-driven virtual reality platforms to transform CT and MRI scans into 3D models for better surgical planning. The University of California, San Francisco, together with IBM Research, has paired machine learning with cellular engineering. Using AI, they created a virtual molecular library, helping engineered immune cells target and eliminate cancer cells. This is done by providing cells with thousands of “command sentences”.
In Australia, the University of Sydney, in collaboration with Pharos Therapeutics, is advancing AI in oncology by developing AI-powered imaging technologies for early detection and cancer treatment. They are leading a $10 million project focused on large-scale 3D imaging trials for melanoma and skin cancer to create next-generation solutions.
In Japan, the National Cancer Center (NCC) integrates AI into CT scan analysis for more accurate detection of lung and gastric cancers. In collaboration with other institutions, NCC participates in initiatives like LC-SCRUMAsia, which focuses on genetic screening of lung cancer patients across Asia. Furthermore, NCC has launched the MONSTARSCREEN-2 project, which uses multi-omics analysis powered by AI to better understand cancer. This approach incorporates wholeexome and whole-transcriptome sequencing to analyse DNA and RNA from tumor tissues, aiming to enhance cancer diagnostics and treatment planning.
In Germany, the German Cancer Research Center (DKFZ) employs AI to analyse large-scale genomic data to advance personalised cancer immunotherapies by identifying tumor-specific “neoepitopes” through mass spectrometry. Charité - Universitätsmedizin Berlin is exploring AI in pathology and leading the EMPAIA project to develop AI-assisted diagnostic imaging solutions. Moreover, they research cancer survivorship.
These global efforts demonstrate the transformative power of AI in cancer care.
Bringing AI to Patients in Ireland: Early Detection and Diagnostics
Over-diagnosis leads to unnecessary treatments, causing side effects, stress, and financial burden. Meanwhile, underdiagnosis delays care. AI helps identify patients who truly need early interventions, reducing both risks. By integrating AI into cancer diagnostics, Ireland is making significant strides toward earlier detection, more precise treatments, and a more efficient healthcare system.
Traditional methods of diagnosing cancer, such as imaging, physical exams, biopsy, and laboratory tests, have long faced limitations. They often struggled to identify early-stage cancers, detect subtle abnormalities, and provide personalised, scalable care. While effective in many cases, these methods rely heavily on human assessment, making them prone to human error and fatigue. For example, X-rays, mammograms, ultrasounds, CT scans, MRI scans, are crucial in detecting tumors, but require radiologists to manually interpret images, which increases the risk of missed diagnoses. Similarly, physical exams, such as self-checks and doctor-led palpation, can help detect lumps
but fail to identify microscopic tumors or asymptomatic cases. Biopsy procedures are accurate but invasive and may not capture the full extent of metastasis. Likewise, blood tests measuring tumor markers lack specificity, making them unreliable as standalone diagnostic tools.
In Ireland, the integration of AI in oncology has evolved, particularly in radiology and pathology:
• The Mater Misericordiae University Hospital in Dublin has become the first public hospital in Ireland to implement AI in Radiology. Since the introduction, over 15,600 scans have been analysed, with 700+ pathologies correctly flagged within 2-3 minutes.
• The University of Limerick (UL) and Dell Technologies have partnered to develop an AI platform and digital twin technology (computational replicas of patients’ cellular environments) for cancer research, enabling simulations to predict disease progression and treatment responses, particularly for B-cell lymphoma.
• The University of Galway’s Insight Centre for Data Analytics has created SKnowGPT, a system enhancing Large Language Models (LLMs) with domain-specific Knowledge Graphs. This improves AI’s ability to answer complex medical queries accurately.
• The Classica project, led by Professor Ronan Cahill and partners, aims to refine AIdriven imaging for real-time cancer surgery decisions using fluorescence imaging.
• Systems Biology Ireland focuses on cellular signaling networks for new cancer therapies. Their work combines multi-omics data, wet-lab experiments, and computational modeling to identify key cancer-related genes to facilitate personalised treatments.
• The CLARIFY project, funded by Horizon Europe, uses AI to analyse data from cancer survivors to improve follow-up care and early detection.
• POI uses computational modeling to develop more accurate diagnostic tests and personalised treatments.
• AI systems like DeepMind and IBM’s Watson for Oncology, used globally and in Ireland, assist radiologists and pathologists by automating
image analysis and tissue sample evaluation. They highlight potential issues, reduce waiting times and improve diagnostic accuracy.
AI is transforming cancer diagnostics in biopsy analysis in Ireland. Deciphex, a Dublinbased company, uses AI-powered platforms like Diagnexia and Patholytix to assist pathologists. These tools improve tissue sample analysis, diagnostic speed, and accuracy, while reducing workloads. Deciphex’s platforms are used by the Health Service Executive (HSE) and National Health Service (NHS) and are expanding into North America.
Additionally, the OncDB platform, developed by Mr. Michael McCarthy at University Hospital Galway, uses AI to analyse clinical trial data, helping professionals stay updated with the latest cancer treatments. The DigiCanTrain project at the University of Galway provides digital skills training for oncology professionals. It integrates AI and digital tools into cancer care education.
Between 2019 and 2022, over ¤106 million was invested in cancer research in Ireland, with contributions from eight funders. However, the prevention category received only ¤2.8 million, or 2.7% of the total funding (Health Research Board, 2024). This underfunding could delay AI integration into cancer management. Prevention programs, such as screening, vaccination, and early diagnostic systems, generate vast amounts of structured, high-quality data for AI analysis, which is crucial for predicting cancer risks and training models. Early interventions are also cost-effective, reducing future healthcare burdens. The Health Service Executive (HSE) is exploring AI for national screening programs like BreastCheck and CervicalCheck. National Screening Service (NSS) is assessing AIdriven models to identify highrisk individuals and prioritise screenings.
Over-treatment can expose patients to unnecessary side effects, while under-treatment risks disease progression. AI aims to address these gaps with tailored, data-driven approaches. Traditional methods of treating cancer like surgery, chemotherapy, and radiation are now complemented by targeted, metabolic, immune, CAR-T therapies, and more. In Ireland,

AI-driven precision medicine is advancing personalised cancer care by analysing genetic data, tumor profiles, patient histories, and identifying biomarkers, to recommend optimal treatments. AI is also crucial in drug discovery, treatment tracking, and clinical trial recruitment - predicting therapy responses and matching patients to appropriate trials.
• In Ireland, institutions like the Irish Cancer Society and Trinity College Dublin are using AI to improve the clinical trial process. AI analyses data from patient records, imaging, and genetic tests to identify trends and support the development of new drugs. This helps researchers discover more effective therapies for challenging cancers like pancreatic and brain cancers.
• The University of Galway is also leading initiatives like the NEO-TIL project. This project develops novel immune therapies for cancers with high mutation rates.
• Led by POI, the cSTAR platform, another notable initiative, creates digital twins to predict patient responses to drug interventions, improving outcomes and minimising side effects.
• Other projects include COLOSSUS, which advances precision medicine for metastatic colorectal cancer.
• There is also 3TR, an IMI2 immunology project, focused on improving disease management across seven immune-mediated diseases.
• The Royal College of Surgeons in Ireland (RCSI) is conducting cutting-edge research on AI’s role in cancer genomics.
Telemedicine in Ireland
Telemedicine is the remote delivery of cancer care, using technology like video consultations, AI platforms (e.g., IBM Watson Health, Babylon Health), and wearable devices (e.g., smartwatches, biosensors). It allows oncologists to monitor patients, provide consultations, and adjust treatments without in-person visits. This is crucial in rural areas with limited healthcare access. The aging population and healthcare professional shortages make remote solutions essential. Telemedicine ensures patients receive timely, continuous care despite geographical barriers. Additionally, the FAITH project in Ireland offers the “AI Angel” app,
which monitors mental health in cancer survivors. This app helps them manage the psychological impact of their diagnosis and treatment.
Ethical Considerations and Who Is Responsible for AI Mistakes in Oncology?
AI integration in oncology is governed by the GDPR in Ireland and across Europe. This ensures patient privacy and data protection. In the US, HIPAA ensures data privacy, while the FDA oversees AI medical devices. Australia follows the Privacy Act 1988, and Asia has diverse regulations like APPI (Japan). When systems make mistakes in healthcare, especially in oncology, accountability can be complex. In Ireland and globally, the responsibility typically falls on a combination of stakeholders. Healthcare providers, such as doctors and specialists, are accountable for interpreting and acting on system recommendations. If AI or technology is involved, the developers or creators of the system could share accountability for ensuring their products are tested, accurate, and reliable. Regulatory bodies also play a role in setting standards and guidelines. Ultimately, when errors occur, accountability may involve both human and technological factors, with each party held responsible for their part in the decision-making process.
Progress in pediatric and adult oncology, driven by research and innovation, offers hope for better outcomes across all ages worldwide. By integrating AI into healthcare, Ireland is wellpositioned to lead in AI-powered oncology. Its promising future is marked by breakthroughs in diagnostic tools, genetic analysis, and precision medicine, supported by ongoing research and collaboration. AI will play a crucial role in early detection, personalised treatments, and innovations like autonomous AI oncologists and robotic surgery. However, the success of these innovations depends on access to quality data, including medical records, imaging scans, and medical tests. Donating such data to research, while ensuring it is anonymised and kept private, will fuel AI advancements. Nations around the world are increasingly adopting AI in the fight against cancer. Collective efforts contribute to the broader global push to improve cancer treatment and patient outcomes.

Written by Professor Jarushka Naidoo, Consultant Medical Oncologist and Dr Janet Moyle, Beaumont RCSI Cancer Centre, Beaumont Hospital, Beaumont

Lung cancer has long been one of the most challenging cancers to treat, often diagnosed at advanced stages when treatment options are limited. However, the treatment landscape is rapidly evolving with the introduction of immunotherapy for early-stage lung cancer. This breakthrough transforms outcomes and offers new hope to patients and their families.
Lung cancer remains one of the leading causes of cancer-related deaths worldwide, with nonsmall cell lung cancer (NSCLC) accounting for 80-85% of cases.1 Traditionally, early-stage NSCLC treatment involved surgery followed by chemotherapy, which is associated with a modest improvement in overall survival of just 5%. Over the past decade,

Dr Janet Moyle
advancements in treatment strategies have aimed to improve survival rates and reduce recurrence risk. Recent clinical trials have led to a paradigm shift, incorporating immunotherapy in both neoadjuvant and adjuvant treatment settings, in combination with chemotherapy in the neoadjuvant setting or given after chemotherapy.
Immunotherapy in Neoadjuvant Treatment: Boosting the Immune Response
Preclinical studies first demonstrated the potential for immunological benefits of neoadjuvant immunotherapy instead of adjuvant immunotherapy. Research showed that immune checkpoint inhibitors (ICIs) administered before surgery enhance the immune response by activating tumour-specific CD8+ T cells while the primary tumour remains intact. These T cells circulate, expand, and infiltrate distant organs, targeting micrometastases and lowering recurrence risk.2 The process also promotes antigen presentation, stimulating naïve T cells and sustaining long-term immune surveillance. It is thought that the optimal immune response may occur with the tumour in situ rather than post surgical resection.
* 1
Pembrolizumab * 1 year
Durvalumab * 1 year
Toripalimab + CT*1 Toripalimab * 1 year
Atezolizumab * 1 year
Tislelizumab * 1 year
Figure: Overview of phase III neoadjuvant chemotherapy + immunotherapy studies for patients with resectable nonsmall cell lung caner
Abbreviations:

KEYTRUDA as monotherapy is indicated for 1L treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥ 50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations.*
KEYTRUDA in combination with pemetrexed and platinum chemotherapy, is indicated for 1L treatment of metastatic NSCLC in adults whose tumours have no EGFR or ALK positive mutations.*
KEYTRUDA in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for 1L treatment of metastatic NSCLC in adults.*
KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥ 1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA.
* Reimbursed
KEYTRUDA in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable non-small cell lung carcinoma at high risk of recurrence in adults.
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with non-small cell lung carcinoma who are at high risk of recurrence following complete resection and platinum-based chemotherapy.
Scan the QR code with your phone to view the KEYTRUDA SPC on medicines.ie
Scan the QR code with your phone to view approved indications for reimbursement on hse.ie
Legal Category: POM
Marketing Authorisation number: EU/1/15/1024/002

Marketing Authorisation holder: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands.
Date of revision: October 2024 © 2025 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.

Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, D18 X5K7 or from www.medicines.ie.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700)

Over the last decade, extensive research has explored combining neoadjuvant immunotherapy with chemotherapy. The CheckMate-816 trial marked a major advancement in this area, becoming the first phase III clinical trial to assess the safety and efficacy of neoadjuvant chemoimmunotherapy versus chemotherapy alone in resectable NSCLC. The study evaluated neoadjuvant nivolumab combined with chemotherapy, without postoperative adjuvant treatment. Results showed that adding immunotherapy reduced the risk of death and distant metastasis. This led to a shift in clinical practice in Ireland in May 2024, with more patients now receiving this innovative treatment approach.3
The IMpower010 trial explored the impact of immunotherapy in the adjuvant setting. This phase III study evaluated adjuvant atezolizumab versus best supportive care in patients with completely resected stage IB-IIIA NSCLC who had received prior platinum-based chemotherapy. The results demonstrated that atezolizumab significantly improved disease-free survival,
particularly in patients with PD-L1-positive tumors.3 These findings, combined with those from CheckMate-816, suggest that a combination of neoadjuvant and adjuvant immunotherapy offers a promising treatment strategy for early-stage NSCLC.
Several phase III clinical trials are have investigated the prognostic effect of perioperative immunotherapy combined with chemotherapy versus chemotherapy alone in resectable non-small cell lung cancer. These trials differed in the characteristics of immunotherapy selection, dosing patterns, and primary endpoints. The figure on page 76 provides a summary of the study characteristics and endpoints.
The results demonstrate that patients with or without pCR may benefit from perioperative immunotherapy plus chemotherapy. CheckMate 816 showed that neoadjuvant nivolumab plus chemotherapy significantly improved event-free survival (EFS) and pathological complete response (pCR) rates, leading to better surgical outcomes and reduced recurrence risk. CheckMate 77T reinforced these benefits, demonstrating prolonged disease-free survival (DFS) and overall survival (OS) with adjuvant nivolumab following neoadjuvant
chemoimmunotherapy.3 KEYNOTE-671 supported perioperative pembrolizumab plus chemotherapy, showing significant improvements in EFS, OS, and pCR, while the AEGEAN trial highlighted durvalumab’s potential in improving pCR and DFS when combined with neoadjuvant chemotherapy and continued postoperatively. The NEOTORCH and RATIONALE 315 trials showed that toripalimab and tislelizumab, respectively, enhanced pCR and DFS when combined with neoadjuvant chemotherapy.
The NADIM phase II trial provided the most impressive evidence of immunotherapy's long-term benefits. It assessed the efficacy of neoadjuvant chemoimmunotherapy followed by adjuvant immunotherapy in patients with resectable stage IIIA NSCLC. At the 5-year follow-up, 65% of patients remained diseasefree, and 69.3% were still alive.4 Notably, no further tumour-related relapses were observed beyond the 29-month mark, suggesting that patients who remain diseasefree beyond three years may be considered cured.
One of the key advantages of immunotherapy over traditional
Following a mixed report card for Ireland from the European Commission Country Cancer profiles, the Irish Cancer Society is calling on the new Minister for Health to prioritise improvements in cancer care. In particular, it is calling for expanded screening services, shorter waiting times for cancer tests and treatment and faster access to new medicines. Every two years, the European Cancer Inequalities Registry publishes data on cancer prevention and care to highlight the strengths, challenges and specific areas of action for each of the 27 EU Member States, Iceland and Norway.
This year’s report card for Ireland shows some positives:
• Ireland outperforms most EU countries in managing key risk factors for cancer, such as tobacco use.
• Alcohol consumption in Ireland has decreased and is slightly under the EU average.
• Consumption of fruit and vegetables is higher here than the EU average.
• The percentage of Irish adults engaging in insufficient physical activity, at 51% in 2022, was significantly lower than the EU average of 70%.
• Of those eligible for breast and cervical cancer screening, participation is higher in Ireland than the EU average.
However, there are many areas of concern:
• Ireland had the second highest rate of new cancer diagnoses among EU countries in 2022.
• While Ireland’s cancer mortality rate declined significantly between 2011 and 2021, it was
chemotherapy is its improved tolerability. While chemotherapy is associated with significant side effects such as nausea, fatigue, and low blood counts, immunotherapy tends to have a more manageable side effect profile. Immune-related adverse effects remain a concern, but overall, the treatment is better tolerated. Additionally, the substantial survival benefits observed in clinical trials suggest that integrating immunotherapy into early-stage NSCLC treatment could lead to a higher cure rate.
With ongoing research and continued clinical trials, the role of immunotherapy in lung cancer treatment is expanding. Future studies will focus on optimizing the combination of immunotherapy with surgery, radiation, and other treatments to maximize patient outcomes. Immunotherapy is shifting the focus from disease management to potential cure, making lung cancer a more treatable condition than ever before. As advancements continue, the prospects for lung cancer patients are becoming increasingly hopeful.
References available on request
still higher than the EU average and the third highest in Western Europe.
• While Ireland has a higher ratio of physicians and nurses per 1,000 new cancer cases than the EU average, it has a shortage of GPs, radiologists, radiation therapists and other key medical personnel.
• The supply of diagnostic equipment, such as MRI and CTI scanners, is significantly lower here than the EU average.
• Vaping rates among Irish 1524 year-olds have increased dramatically, from 1% in 2015 to 10% in 2023.
• Irish patients have access to a narrower range of new oncology medicines than the EU average. The report also warns that the cost of cancer care in Ireland is set to
rise. Per capita health expenditure on cancer care is expected to grow by 80% in Ireland between 2023 and 2050, compared to 59% in the EU27.
Averil Power, CEO of the Irish Cancer Society said, “While we can be proud of the leadership Ireland has shown in relation to tobacco control, we are still far off our national target of a 5% daily smoking rate. The significant increase in youth vaping highlighted by this report is also hugely worrying. Not only is vaping harmful in itself, it is also a proven gateway to smoking tobacco. Irish obesity levels are also a concern, with over half of Irish adults classified as obese or overweight in 2022, albeit a slight reduction since 2017. Previous Government investment in tobacco control and health promotion has paid dividends but more can be done to make the healthy choice the easy choice.”

Written by Dr Olwyn Conlon, Dermatology Registrar1, Professor Mary Laing, Consultant Dermatologist1
1Department of Dermatology, University Hospital Galway
1. Introduction
Skin cancer is the most common cancer in Ireland with over 13,000 new cases diagnosed every year.1 Primary cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer. CSCC is a malignant tumour arising from keratinising cells of the epidermis or its appendages, and as such is considered a keratinocyte or non-melanoma skin cancer (NMSC).
According to the National Cancer Registry Ireland, from 1984-2010, overall incidence of cSCC has increased by 263%.
While Basal Cell Carcinoma (BCC) is a more prevalent NMSC, cSCC poses a greater risk due to its potential for local invasion, lymph node involvement and in rare cases, metastasis. While often treatable, cSCC can become aggressive, leading to significant morbidity and mortality if not managed appropriately.
In Ireland, the incidence of cSCC has been steadily rising, in line with global trends. Between 2011 and 2015, nearly 11,000 invasive skin cancers were diagnosed annually in Ireland. Of these, 3,000 cases per year (approximately 27%) were cSCCs. The rise in keratinocyte cancer is attributed to several factors, including greater cumulative sun exposure, an aging demographic and increased awareness leading to higher detection rates.2
2. Pathophysiology
As mentioned cSCC arises from the malignant transformation of keratinocytes in the epidermis. Recent studies have identified key molecular alterations driving cSCC development. Mutations in tumour suppressor genes such as TP53 and CDKN2A disrupt cell cycle regulation leading to uncontrolled cellular proliferation, while aberrant signalling in the P13K/AKT/ mTOR pathway has also been implicated in tumour growth and survival. Genetic polymorphisms in the Methylenetetrahydrofolate
Reductase chloride transport protein 6 (MTHFR CLCN6) gene has been associated with keratinocyte skin cancer in a cohort of renal transplant recipients.3 Epigenetic aberrancies are also evident in cSCC in renal transplant recipients.4
UV radiation induces DNA damage due to the formation of cyclobutene pyrimidine dimers which can result in carcinogenesis. The UV Index is a standardized measure of the intensity of UV radiation, which ranges from 0 (low) to 11+ (extreme). The higher the number, the greater the potential for UV skin damage. The UV Index in Ireland typically ranges between 1 and 7. During the winter months (November–February), the UV Index is usually low (0-2).
In the summer months (May–August), the UV Index often reaches 5-7, especially on clear days between 11 AM and 3 PM. Many people in Ireland assume that cloudy weather reduces UV risk, but over 80% of UV rays can penetrate cloud cover. Reflection from surfaces such as water, sand and snow can further increase UV exposure.
The Celtic skin type (Fitzpatrick phototype I-II) predominant in the Irish population places individuals at greater risk of sunburn with increased susceptibility to UV damage. This skin type coupled with prolonged and often unprotected sun exposure (especially in those who work outdoors) results in a population at higher risk of developing cSCC. Another at risk group is immunosuppressed organ transplant recipients. CSCC is the predominant skin cancer type in renal transplant patients, who have a 65-250 fold increased risk of developing cSCC compared to the general population. CSCC in organ transplant patients also has a more aggressive clinical course, a greater tendency to metastasise and a higher mortality compared to the general population. The increasing use of immunosuppressive therapies


in additional patient cohorts is also contributing to an overall increasing burden of cSCC.
Certain ocular iris patterns in the general population are associated with keratinocyte cancers such as a blue periphery, blue collarette and an absence of freckles.5 Meanwhile in immunosuppressed renal transplant recipients, blue iris periphery, light brown collarette and iris freckling confer an independent risk for keratinocyte skin cancer.6 In addition, certain medications have been implicated in increasing the risk of developing cSCC such as voriconazole used to treat aspergillosis, and hydrochlorothiazide, a commonly prescribed diuretic.
During the 1980s and 1990s, dermatologists in Ireland began to observe an increase in cSCC cases in younger individuals and those with occupational and higher cumulative UV exposure, i.e. farmers, fishermen, construction workers and sports coaches. At the same time, improved access to dermatological services led to better diagnosis, documentation, and reporting of skin cancers, contributing to the apparent rise in incidence.
In 2024 the Irish Association of Dermatologists through the EADV Representative for Ireland, Professor Mary Laing, supported a global call to action to protect outdoor workers from skin cancer by solar UVR exposure at the 2023 EADV congress.
Initiatives such as the Irish Cancer Society’s SunSmart campaign emphasized the importance of sunscreen use, protective clothing and regular skin checks, particularly for high-risk groups. Despite these efforts, many Irish people continue to underestimate the impact of UV exposure, leading to sustained increases in cSCC incidence. A 2007 study examining Irish attitudes and perceptions regarding skin cancer and sun protection by Jones et al. found that 90% of participants knew that sun exposure was the major risk factor for skin cancer, and 95% knew that sun beds were not a safe way to tan. Despite this knowledge <20% used regular sunscreen, and 30% had used or were currently using sun beds in order to tan.7
Ireland has the highest per capita use of fake tan globally. Unlike UV-induced tanning, which poses health risks, fake tan offers a

that enhances the visualisation, early detection, and differentiation of cSCC from other skin conditions.

• Tumour diameter > 20mm
• Tumour depth > 4mm
• Tumour extending beyond dermis into or through subcutaneous fat
• Perineural invasion
• Poor differentiation
• Desmoplastic subtype
• Immunosuppression
Treatment decisions are tailored to the individual patient, considering staging, the patient's overall health and their preferences.
safer alternative. The popularity of fake tan in Ireland is attributed to cultural beauty standards favouring tanned skin amid the country's predominantly fair-skinned population. Patients presenting with fake tan to pigmented lesion clinics, can lead to difficulties in accurate diagnosis which can result in unnecessary appointment rescheduling.8
• Central Keratin manifesting as a yellow/ white clump
Common Dermoscopic features of non-pigmented cSCC as outlined by Professor Harald Kittler of the Medical University of Vienna (9), include:
• Blood Spots which are small, round, dark red to black structures that result from haemorrhage into the keratin mass
• Atypical Keratinocyte Morphology with irregular, pleomorphic cells and increased nuclear-to-cytoplasmic ratios
• Disruption of the Normal Epidermal Architecture with loss of the regular honeycomb pattern in the epidermis
• White Circles which appear as well-defined, round structures and are considered a hallmark of cSCC. They correspond to hyper granulosis of the infundibular epidermis
4. Diagnosis
• Pink or Red Background often seen in poorly differentiated or rapidly growing tumours
• White Structureless Areas which lack specific patterns, presenting as homogeneous white zones, associated with fibrosis
In cases of pigmented cSCC, dermoscopy may show
• Increased vascularity and Inflammatory Infiltrates
5. Treatments
In advanced cSCC, further imaging may be required to assess disease extent. CT evaluates bone, soft tissue, and cervical lymph node involvement, while MRI is useful for head and neck spread and perineural invasion. Radioisotope bone scans detect suspected bony metastases.
I. Surgery
CSCC diagnosis in Ireland is based on clinical examination and histopathology. CSCC lesions typically present as a firm, erythematous nodule or plaque with a crusted surface. Lesions may ulcerate and are commonly found on sun-exposed sites such as the face, ears, neck and hands. Patients typically complain of a non-healing ulcer which may or may not be tender in a sun-exposed area. Dermoscopy remains a valuable, non- invasive diagnostic tool that enhances the visualisation, early detection, and differentiation of cSCC from other skin conditions.
• Looped or Coiled Vessels
• Central Keratin manifesting as a yellow/ white clump
• Diffuse homogeneous blue pigmentation or blue-grey pigmented granular structures, often irregularly distributed across the lesion
• Blood Spots which are small, round, dark red to black structures that result from haemorrhage into the keratin mass
Diagnosis is established histologically with CSCC staging following the AJCC 8th Edition TNM system, which classifies tumours based on size, depth, nerve involvement, lymph node spread and distant metastasis.
• Pink or Red Background often seen in poorly differentiated or rapidly growing tumours
In cases of pigmented cSCC, dermoscopy may show
An additional diagnostic tool available in certain centres includes Reflectance Confocal Microscopy (RCM) which can provide real-time, high-resolution images of the epidermis and superficial dermis at near histologic resolution. Studies have shown that cSCC exhibits specific confocal features such as:
According to the Scottish Intercollegiate Guidelines Network (SIGN) guidelines, the choice of treatment for cSCC may be discussed in a multidisciplinary team meeting where any of the following high-risk features are present:
• Diffuse homogeneous blue pigmentation or blue-grey pigmented granular structures often irregularly distributed across the lesion
Common Dermoscopic features of non-pigmented cSCC as outlined by Professor Harald Kittler of the Medical University of Vienna9, include:
• White Circles which appear as well-defined, round structures and are considered a hallmark of cSCC. They correspond to hyper granulosis of the infundibular epidermis
• White Structureless Areas which lack specific patterns, presenting as homogeneous white zones, associated with fibrosis
• Looped or Coiled Vessels
For clinically well-defined, low risk tumours < 2cm in diameter, surgical excision with a minimum 4mm margin around the tumour border is appropriate and would be expected to completely remove the primary tumour mass in 95% of cases. For tumours > 2cm in diameter, classified as moderately, poorly, or undifferentiated and extending into the subcutaneous tissue or those on the ear, lip, scalp, eyelids or nose, such tumours should be removed with a wider margin of 6mm or more. Mohs micrographic surgery is particularly beneficial for high-risk cSCCs, located in areas where preserving cosmetic and functional integrity is important i.e., on the face, ears, or hands and those cSCCs with an indistinct clinical border, that are large or rapidly growing, that recurred after


previous treatment and/or exhibit aggressive histological features.
tumour cells, or taxanes (Paclitaxel, Docetaxel) which disrupt microtubule function, inhibiting mitosis.
protection standards.12 The photoprotective standard of clothing is measured as the ultraviolet photoprotective factor (UPF), the UPF rating provides information on how much UV will pass through unstretched dry material and measures skin erythema at various UV radiation doses, analogous to the SPF of sunscreen. Hat material with a UPF rating of 30 would allow 1/30th (3.3%) of UV falling on its surface to pass through it, blocking 96.7% of UV. Kearney et al. found that only 35% of the UK's largest online retailers stock UPF-rated clothing.13
The procedure involves the precise, layer-by-layer removal of cancerous tissue with each layer examined microscopically in realtime to ensure complete cancer eradication while minimizing the loss of surrounding healthy tissue. The immediate microscopic examination of excised tissue ensures that all cancerous cells are removed during the initial procedure, reducing likelihood of recurrence. Mohs surgery offers cure rates up to 99% for primary cSCCs, making it one of the most effective treatments available.
This involves applying a photosensitizing agent to the skin, which is activated by a specific wavelength of light to produce reactive oxygen species that selectively destroy cancerous or pre-cancerous cells. PDT requires 1-2 treatment sessions at 1–2-week intervals and may be preferred if there are larger lesions covering a wider BSA.
V. Systemic Treatments
II. Radiotherapy
The National Skin Cancer Prevention Plan 2023-2026 has re-emphasized the importance of public education on sun safety and risks associated with UV radiation. Campaigns aim to reduce cSCC incidence by encouraging protective behaviours and regular self-skin-checks.
Mohs micrographic surgery is particularly beneficial for high-risk cSCCs, located in areas where preserving cosmetic and functional integrity is important i.e., on the face, ears, or hands and those cSCCs with an indistinct clinical border, that are large or rapidly growing, that recurred after previous treatment and/or exhibit aggressive histological features.
The metastatic rate of cSCC is 2-5% in the general population. However cSCC in high risk sites such as the lip or ear has a 10-20% metastatic rate. Where cSCC has metastasized or is not amenable to local treatments, systemic therapies may be considered.
Skin cancer is largely preventable, with UVR from the sun or sunbeds responsible for 90% of cases. While sunscreen plays a role in photoprotection, it alone is not enough to prevent keratinocyte cancer. Instead, it should be used alongside protective clothing and shade for optimal protection. There are a number of factors which may limit sunscreen use. One Irish observational study highlighted that tinted sunscreen may have poorer cosmetic acceptability for individuals with darker skin tones due to a white caste. Sunscreens with zinc oxide or titanium dioxide can leave a white residue, making them less appealing for people with darker skin tones.10
The integration of immunotherapies like cemiplimab, offer new avenues for patients with metastatic or inoperable tumours. Ongoing research into combination therapies holds promise for improved outcomes.
The procedure involves the precise, layer-by-layer removal of cancerous tissue with each layer examined microscopically in real-time to ensure complete cancer eradication while minimizing the loss of surrounding healthy tissue The immediate microscopic examination of excised tissue ensures that all cancerous cells are removed during the initial procedure, reducing likelihood of recurrence. Mohs surgery offers cure rates up to 99% for primary cSCCs, making it one of the most effective treatments available.
Radiotherapy uses high-energy radiation to target and destroy cancer cells. It is employed when surgery is not feasible or as an adjunct treatment to reduce risk of recurrence. It may also be considered for patients with large tumours, perineural invasion or when surgical margins are positive.
III. Cryotherapy
Cryotherapy involves freezing cancerous tissue with liquid nitrogen, causing cells to die, and can be used to treat Squamous cell carcinoma in situ (SCCIS)/ Bowen’s disease which is where malignant cells are confined to the epidermis, and are small and superficial.
IV. Topical Treatments
• Immunotherapy: The European Medicines Agency authorized cemiplimab (Libtayo) as monotherapy for adult patients with metastatic or locally advanced cSCC who are not candidates for curative surgery or curative radiation. The HSE approved its reimbursement effective from October 2024. Cemiplimab is a monoclonal antibody that works as an immune checkpoint inhibitor targeting programmed death receptor-1 (PD-1). In December 2024, the FDA approved cosibelimab (Unloxcyt) for similar indications, expanding therapeutic options.
In Ireland, keratinocyte cancer currently affects 1 in 6 men and 1 in 9 women over their lifetimes. Men are more commonly affected, likely due to greater lifetime head and neck exposure to UV radiation. Older adult males are at higher risk of developing head and neck skin cancers, presenting at later stages and have worse outcomes overall. Hynes et al. examined males attending Dermatology services in the West of Ireland, they identified that most patients reported wearing hats and SPF, however a minority wear SPF on their lips and often Irish men wear a hat type (baseball cap) that would have too small a brim to provide adequate photoprotection to the head and neck.11
This January marks the 10th anniversary of the commercial sun bed ban in Australia. Recognizing the risks associated with UV radiation, the Irish government has also taken steps to regulate sunbed use. The Public Health (Sunbeds) Act 2014 prohibits the sale or hire of sunbeds to individuals under 18 and imposes strict regulations on sunbed businesses. In July 2024, Taoiseach Micheál Martin expressed strong support for a complete ban on sunbeds, citing compelling evidence linking their use to increased skin cancer risk.
Radiotherapy uses high-energy radiation to target and destroy cancer cells. It is employed when surgery is not feasible or as an adjunct treatment to reduce risk of recurrence. It may also be considered for patients with large tumours, perineural invasion or when surgical margins are positive.
Similarly, for SCCIS/ Bowen’s disease, topical treatments may be appropriate:
• Chemotherapy Creams: Include 5 fluorouracil cream (Efudix) and imiquimod 5% cream (Aldara).
• Photodynamic Therapy (PDT):
• Chemotherapy: For locally advanced or metastatic cSCC, systemic chemotherapy may be used in combination with surgery, radiotherapy, or immunotherapy. Regimens may be platinum-based (Cisplatin, Carboplatin) which cause DNA crosslinking leading to apoptosis in rapidly dividing
When considering photoprotection campaigns, fashion trends regarding hat types should be considered. The World Health Organisation (WHO) advocates for the use of photoprotective clothing including wide-brimmed hats, tightly woven clothing, and wraparound style sunglasses that provide 100% UV-A and UV-B protection to prevent the effects of UVR. In Ireland there is frequently poor availability in shops of hats meeting sun
While the number of cases of cSCC diagnosed annually in Ireland is projected to double by 2040, advancements in treatment and growing awareness offer hope for the future. Proactive prevention measures, such as targeted public education campaigns and enhanced early detection efforts, combined with innovative immunotherapies, are paving the way for improved outcomes. With a unified commitment to sun safety, research, and equitable access to care, Ireland is wellpositioned to tackle this growing health challenge and ensure better care for patients nationwide.
References available on request Dermatoscopic image courtesy of Dr Karen Reidy1
Cryotherapy involves freezing cancerous tissue with liquid nitrogen, causing cells to die, and can be used to treat Squamous cell carcinoma in situ (SCCIS)/ Bowen’s disease which is where malignant cells are confined to the epidermis, and are small and superficial.

Mater Private Network is proud to announce the successful integration of the cutting-edge FARAVIEW™ Software, utilised in the treatment of Atrial fibrillation (AFib), to its Dublin centre of excellence. As one of just seven centres granted early access to this revolutionary technology under limited market release, Mater Private Network is driving the future of cardiac care in Ireland, benefiting hundreds of patients nationwide.
This milestone follows Mater Private Network’s selection as one of only three centres globally to take part in the FARAVIEW technology study into the treatment of AFib, highlighting the Network’s leadership in cardiac electrophysiology. Leading this initiative in Ireland are Prof. Gábor Széplaki, Head of Cardiac Electrophysiology, and Dr. Noel Fitzpatrick, Consultant Cardiologist at Mater Private Network in Dublin.
Mater Private Network team, lead by Prof. Gábor Széplaki & the team from Boston Scientific celebrate the integration of the FARAVIEW technology in the MPN Cath Lab, Dublin
Atrial fibrillation (AFib) is the most common irregular heart rhythm, with an estimated 8 million patients living with this condition in Europe, suffering from symptoms such as fatigue, palpitations and breathlessness, depending on the severity of the condition. Minimally invasive, innovative technologies such as the FARAPULSE Pulsed Field Ablation System may change the course of treatment for many in the future.
Professor Gábor Széplaki explains, “At Mater Private Network, we participate in trials of innovative new technology such as this, with the goal of setting new standards in Irish cardiac care and we are delighted with this result. The integration of FARAVIEW mapping with the FARAPULSE PFA System allows us to offer safer, faster, and more precise treatments for those suffering from AFib. As one of the few institutions with access to this cutting-edge development, this technology marks a new era in AFib care in Ireland. We are proud to be a part of that journey,
The Medical Council has launched a public consultation on draft Rules for the establishment of subcommittees of the Preliminary Proceedings Committee (PPC).
The role of the Medical Council, as the regulator of doctors in Ireland, is to protect the public by ensuring high standards of professional conduct, performance, and education among doctors. One of the ways it carries out this role is to investigate complaints raised about the health, performance and conduct of doctors in Ireland.
Section 81 of the Regulated Professions (Healthand Social Care) (Amendment) Act 2020 will amend Section 11 of the Medical Practitioners Act 2007 to provide
for the establishment of subcommittees of the PPC. This public consultation is requesting feedback from stakeholders on one element of the amendment – that the Chair of the PPC or a nominated member of the PPC will have the power to create subcommittees (or smaller groups) to review and provide opinion to the Medical Council on complaints received.
When a complaint is made to the Medical Council, it is handled initially by the PPC. The PPC’s function is to give initial consideration to complaints against doctors in order to identify the cases which raise serious concerns and where it is necessary to take further action.
Its role is to conduct a preliminary assessment of the complaint to determine whether there is sufficient cause to warrant further action being taken. If the PPC decides that a complaint warrants further action, it may refer the complaint to the Fitness to Practise Committee.
This stakeholder consultation provides an opportunity for doctors, members of the public, individuals and organisations to submit comments and suggestions on the draft Rules relating to the establishment of PPC subcommittees. Feedback via the online survey is welcomed until Friday 7th March 2025.
ensuring our patients receive world-class, minimally invasive solutions that significantly improve their quality of life.”
Since adopting the FARAPULSE™ PFA System in 2022, the Electrophysiology team at Mater Private Network has treated over 1,400 patients, targeting cardiac tissue while preserving surrounding structures. The introduction of FARAVIEW™ integrated mapping enhances existing technologies, allowing for precise visualisation of the FARAWAVE NAV catheter on the OPAL HDx Mapping system. This system also reduces the need for X-ray exposure and improves procedural workflow, accuracy, and outcomes for patients.
During the pre-launch trial, the Mater Private Network team completed 10 cases under the NAVIGATE-PF Study. Completion of that first in men trial was required to get approval for the device from the competent authorities in the US and Europe. Globally, over 200,000 cases of AFib have been treated with FARAPULSE PFA, including 2,100 FARAVIEW cases in the USA.
Mater Private Network is a national leader in cardiology services in Ireland, known for its commitment to medical innovation and excellence in patient care. To learn more about the cutting-edge services available across the Network, please visit www.materprivate.ie
Dr Suzanne Crowe, President of the Medical Council, encouraged stakeholders to participate in the survey: “The Regulated Professions (Health and Social Care) (Amendment) Act 2020 introduces amendments to existing legislation governing the regulation of those practising in healthcare, including doctors, nurses, pharmacists, dentists and other health and social care professionals.
“The Medical Council carries out regular public consultations so that the opinion and input of our stakeholders is considered in the work we are undertaking. I would invite anyone who has an interest in the remit of the Medical Council to take part.”
Up to half of all people living with Alzheimer’s Disease in Ireland remain undiagnosed. A new blood test may have the potential to transform patient care, allowing for better diagnosis, earlier interventions and more targeted treatments
Researchers at Trinity College, the Tallaght Institute of Memory and Cognition and St James’s Hospital, Dublin are exploring the ability of a new blood test, plasma p-tau217, to detect Alzheimer’s Disease (AD). This test could potentially replace the current diagnostic method, a lumbar puncture/spinal tap (which is invasive and poses risks and challenges) in over half of patients with early symptoms, thus allowing more patients to be diagnosed more accurately and with greater efficiency.
The study is published in the journal Alzheimer’s & Dementia: Diagnosis, Assessment and Disease Monitoring.
In Ireland, over 60,000 people live with dementia, with Alzheimer’s Disease accounting for about 70% of cases. In order to enable accurate diagnosis, biomarkers are currently measured in cerebrospinal fluid (CSF) obtained using a diagnostic lumbar puncture (LP) procedure. An accurate clinical biological diagnosis of Alzheimer Disease is valued by patients and aids future medical care. Of those in Ireland currently living with Alzheimer’s Disease, up to half do not have a formal diagnosis, highlighting the need for improved diagnostic methods which are accurate and can be used at scale.
The study is one of the first in Europe to examine the “realworld” performance of one of the leading automated blood
Dr
Jakub Gajewski named researcher of the year
tests for Alzheimer’s Disease, plasma p-tau217, in patients with mild symptoms undergoing assessment in a specialist memory service. 148 patients attending Tallaght University Hospital (TUH) generously donated blood and cerebrospinal fluid (CSF) samples at the time of their LP, enabling researchers to directly compare new blood tests to established CSF biomarkers. Crucially, this was performed using fully-automated technology (Lumipulse®), which already exists in clinical diagnostic laboratories. The use of a fully-automated system increases reliability over time in the laboratory as well as reliability between different laboratories.
The Trinity College study was a collaboration between the Immunology Department at St James’s Hospital (led by Chief Medical Scientist Dr Jean Dunne & Consultant Immunologist Professor Niall Conlon) and the Tallaght Institute of Memory and Cognition at Tallaght University Hospital (Dr Adam Dyer, Specialist Registrar in Geriatric Medicine & Professor Sean Kennelly, Director). The study found that measuring plasma p-tau217 using a fully-automated system, was >90% as accurate as results obtained from LP. Integrating the blood test into clinical pathways could potentially avoid the need

for over half of diagnostic LPs. This has clear implications for the diagnosis and management of early Alzheimer Disease.
From their results, the research team believe that this new blood test could replace over half of the 150-200 diagnostic LP procedures that they currently carry out in the Tallaght Institute of Memory and Cognition every year.
Dr Jean Dunne, Chief Medical Scientist, Department of Immunology, St James’s Hospital and Trinity Translational Medicine Institute (TTMI) said:
“This blood test is not available currently in Ireland and the findings from this research will lend support to making it available in the future. This ‘translation’ from a research to a diagnostic test is dependent on the scientists, the clinical teams and the support from hospital management.
Using this automated analyser the scientists at St James’s will be able to deliver a reliable and reproducible diagnostic test result. The quality assurance carried out in the diagnostic laboratory includes comparison of results achieved to those reported internationally. All of this research will benefit the patient and the clinical teams and combines the research and diagnostic expertise to deliver a world class, patient centred service.”
Dr Adam Dyer, Specialist Registrar in Geriatric Medicine & Clinical Lecturer in Medical Gerontology, Trinity College Dublin said, "This study brings us one step closer to using diagnostic blood tests, such
as plasma p-tau217, to assist in the clinical-biological diagnosis of early Alzheimer’s Disease. Importantly, this research assessed plasma p-tau217 using fullyautomated technology already available in clinical laboratories and used samples from a "realworld" clinical cohort.
“We are incredibly grateful to the 148 patients who contributed to this research by donating their blood and cerebrospinal fluid samples at time of their diagnostic workup in the Tallaght Institute of Memory and Cognition."
Professor Sean Kennelly, Tallaght Institute of Memory and Cognition in Tallaght University Hospital & Clinical Associate Professor, Trinity College Dublin, added,
“Our research, conducted in collaboration with colleagues from Trinity College Dublin and St. James Hospital Dublin, represents a further significant step forward in the early and accurate diagnosis of Alzheimer’s disease. By demonstrating the clinical utility of blood-based biomarkers, we are moving closer to a future where diagnosing this condition is less invasive, more accessible, and available to a broader population.
“This has the potential to transform patient care, allowing for better diagnosis, earlier interventions and more targeted treatments. At the Tallaght Institute of Memory and Cognition, we are extremely grateful to all our patients who contributed to this project, and remain committed to advancing research that improves the lives of those affected by Alzheimer’s and other cognitive disorders.”
For postmenopausal women at high risk of fracture
To reduce the risk of fracture2 and to provide continuous BMD* gains over the long term1,5
Osteoporosis is a chronic long-term condition that requires ongoing continuous treatment 6-10†

*Bone Mineral Density. † Prolia should not be stopped without considering alternative treatment in order to prevent rapid bone mineral density loss and a potential rebound in vertebral fracture risk.7
References: 1. Prolia® (denosumab) SmPC. 2. Cummings SR, et al. N Engl J Med. 2009;361:756-65. 3. Holzer G, et al. J Bone Miner Res. 2009;24:468-74. 4. Poole K et al. J Bone Miner Res. 2015;30:46-54. 5. Bone HG, et al. Lancet Diabetes Endocrinol. 2017;5:513-23. 6. HSE Living Well. Available at: https://www2.hse.ie/living-well/exercise/being-active-health-condition/being-active/ Accessed 10th February 2024. 7. Tsourdi E, et al. Bone. 2017;105:11-7. 8. Hernlund E, et al. Arch Osteoporos. 2013;8:136. 9. Kanis, JA, et al. Osteoporos Int. 2019;30:3-44. 10. Brown JBMR 2013 Vol28 pp746-752.
PROLIA® (denosumab) Brief Prescribing Information. Please refer to the Summary of Product Characteristics (SmPC) before prescribing Prolia. Pharmaceutical Form: Pre-filled syringe with automatic needle guard containing 60 mg of denosumab in 1 ml solution for injection for single use only. Contains sorbitol (E420). Indication: Treatment of osteoporosis in postmenopausal women at increased risk of fractures. Dosage and Administration: 60 mg Prolia administered as a subcutaneous injection once every 6 months. Patients must be supplemented with calcium and vitamin D. No dosage adjustment required in patients with renal impairment. Prolia should not be used in children aged < 18 years because of safety concerns of serious hypercalcaemia. Give Prolia patients the package leaflet and patient reminder card. Re-evaluate the need for continued treatment periodically based on the benefits and potential risks of denosumab on an individual patient basis, particularly after 5 or more years of use. Contraindications: Hypocalcaemia or hypersensitivity to the active substance or to any of the product excipients.
Special Warnings and Precautions: Traceability: Clearly record the name and batch number of administered product to improve traceability of biological products. Hypocalcaemia: Identify patients at risk for hypocalcaemia. Hypocalcaemia must be corrected by adequate intake of calcium and vitamin D before initiation of therapy. Clinical monitoring of calcium levels is recommended before each dose and, in patients predisposed to hypocalcaemia, within 2 weeks after the initial dose. Measure calcium levels if suspected symptoms of hypocalcaemia occur.
Renal Impairment: Patients with severe renal impairment (creatinine clearance < 30 ml/min) or receiving dialysis are at greater risk of developing hypocalcaemia. Skin infections: Patients receiving Prolia may develop skin infections (predominantly cellulitis) requiring hospitalisation and if symptoms develop then they should contact a health care professional immediately. Osteonecrosis of the jaw (ONJ): ONJ has been reported rarely with Prolia 60 mg every 6 months. Delay treatment in patients with unhealed open soft tissue lesions in the mouth. A dental examination with preventative dentistry and an individual benefit:risk assessment is recommended prior to treatment with Prolia in patients with concomitant risk factors. Refer to the SmPC for risk factors for ONJ. Patients should be encouraged to maintain good oral hygiene, receive routine dental check-ups and immediately report oral symptoms during treatment with Prolia. While on treatment, invasive dental procedures should be performed only after careful consideration and avoided in close proximity to Prolia administration. The management plan of patients who develop ONJ should be set up in close collaboration between the treating physician and a dentist or oral surgeon with expertise in ONJ. Osteonecrosis of the external auditory canal: Osteonecrosis of the external auditory canal has been reported with Prolia. Refer to the SmPC for risk factors. Atypical femoral fracture (AFF): AFF has been reported in patients receiving Prolia. Discontinuation of Prolia therapy in patients suspected to have AFF should be considered pending evaluation of the patient based on an individual benefit risk assessment. Longterm antiresorptive treatment: Long-term antiresorptive treatment may contribute to an increased risk for adverse outcomes such as ONJ and AFF due to significant suppression of bone remodelling. Concomitant medication:
Patients being treated with Prolia should not be treated concomitantly with other denosumab containing medicinal products. Warnings for Excipients: Patients with rare hereditary problems of fructose intolerance should not use Prolia. Interactions: Prolia did not affect the pharmacokinetics of midazolam, which is metabolized by cytochrome P450 3A4 (CYP3A4). There are no clinical data on the co-administration of denosumab and hormone replacement therapy (HRT), however the potential for pharmacodynamic interactions would be considered low. Pharmacokinetics and pharmacodynamics of Prolia were not altered by previous alendronate therapy. Fertility, pregnancy and lactation: There are no adequate data on the use of Prolia in pregnant women and it is not recommended for use in these patients. It is unknown whether denosumab is excreted in human milk. A risk/benefit decision should be made in patients who are breast feeding. Animal studies have indicated that the absence of RANKL during pregnancy may interfere with maturation of the mammary gland leading to impaired lactation post-partum. No data are available on the effect of Prolia on human fertility. Undesirable Effects: The following adverse reactions have been reported: Very common (≥ 1/10) pain in extremity, musculoskeletal pain (including severe cases). Common (≥ 1/100 to < 1/10) urinary tract infection, upper respiratory tract infection, sciatica, constipation, abdominal discomfort, rash, alopecia and eczema. Uncommon (≥ 1/1000 to < 1/100): Cellulitis, ear infection and lichenoid drug eruptions. Rare (≥ 1/10,000 to < 1/1,000): Osteonecrosis of the jaw, hypocalcaemia (including severe symptomatic hypocalcaemia and fatal cases), atypical femoral fractures, and hypersensitivity (including rash, urticaria, facial swelling, erythema and anaphylactic reactions). Very rare (< 1/10,000): Hypersensitivity vasculitis. Please consult the Summary of Product Characteristics for a full description of undesirable effects. Pharmaceutical Precautions: Prolia must not be mixed with other medicinal products. Store at 2°C to 8°C (in a refrigerator). Prolia may be exposed to room temperature (up to 25°C) for a maximum single period of up to 30 days in its original container. Once removed from the refrigerator Prolia must be used within this 30 day period. Do not freeze. Keep in outer carton to protect from light. Legal Category: POM. Presentation and Marketing Authorisation Number: Prolia 60 mg: Pack of 1 pre-filled syringe with automatic needle guard; EU/1/10/618/003. Price in Republic of Ireland is available on request. Marketing Authorisation Holder: Amgen Europe B.V., Minervum 7061, NL-4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. Prolia is a registered trademark of Amgen Inc. Date of PI preparation: May 2022 (Ref: IE-PRO-0322-00006)
Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse reactions/events should also be reported to Amgen Limited on +44 (0)1223 436441 or Freephone 1800 535 160. IRL-162-0124-80001 | Date of preparation: March 2024
Although osteoporosis is often described as the ‘silent epidemic’, the alarm bells for a crisis in the management of osteoporotic fractures continue to sound very loudly.
There is currently a high prevalence of osteoporosis and low bone mass in the Irish population, a feature that is set to grow in accordance with our aging population. The consequences of an osteoporotic fracture, particularly a hip fracture, can be bleak, often leading to longer term disability and increased mortality. The challenge of course, is to identify the people at risk, before a fracture occurs, and to implement an appropriate treatment strategy to reduce fracture risk. Given that successful treatment strategies are available, the solution may seem straight-forward. However, current research shows that there is a large treatment gap, with many people at risk not receiving the treatment they need. While there are various factors that contribute to this treatment gap, lack of timely diagnosis is known to play a role.
In the identification of fracture risk, DXA (Dual X-Ray Absorptiometry) is the gold standard for the measurement of bone mass density (BMD) and the diagnosis of osteoporosis. Indeed, BMD, as determined by DXA, is the single best predictor of fracture. However, with limited DXA services, and an aging population, how can we ensure that the ‘correct’ people are referred for DXA, so as to optimise efficiency of resources, while providing care and prevention for the people most at risk? Research shows that DXA is most beneficial when used to target people with known risk factors, such as advancing age, low body weight and use of glucocorticoid medications. Indeed, the International Society for Clinical Densitometry (ISCD) provide very clear guidelines on who should be referred for DXA https://iscd.org/officialpositions-2023/. Their position is that all women aged 65 or older, and men aged 70 or older, are appropriate candidates for DXA, as well as certain other cohorts of the population with clinical rationale. However, despite this awareness, a mismatch persists within the system, with many people, sadly, missing the opportunity for appropriate preventative care. Although a national screening programme would provide appropriate and equal access for
all, this has not yet materialised for Ireland, despite long-standing calls from academics and clinicians. While Fracture Liaison Services have made immense progress in improving the identification of people at risk of secondary fractures, the ultimate goal is to identify risk earlier and avoid fracture in the first instance.
In the midst of this quandary, research forges ahead to develop useable and innovative approaches for the improved identification of people at risk of osteoporotic fracture. Some of these approaches use predictive algorithms based on known risk factors. The FRAX® fracture risk assessment tool https://frax. shef.ac.uk/FRAX/index.aspx, first launched at the University of Sheffield in 2008, is the best known of these, with its use in Ireland known to have increased between 2011 and 2019. As well as age, sex and weight, it currently includes 8 other risk factors, with the additional option to include BMD, if available, for more accurate predictions. With the more recent launch of FRAXplus®, development is ongoing to refine calculations and to keep apace of emerging research. As many different risk factors for osteoporosis have been identified, accurate prediction of fracture risk is potentially a complex task. However, even simple calculations, such as the Osteoporosis Self Assessment Tool (OST), which considers only age and body weight, have been shown to have high predictive value for use in point-of-care settings. Tools such as these have utility, not only in the clinical decisionmaking process of referral for DXA, but in and of themselves, for the identification of men and women at various stages of life, who may benefit from evidencebased lifestyle, behavioural, and possibly even appropriate pharmaceutical strategies, to maintain bone mass as they age. Another avenue of research is the potential use of bioimpedance analysis for the estimation of bone mass. Bioimpedance analysis is a safe, relatively low cost and convenient technology. It is non-invasive, and takes less than a minute to provide a body composition assessment with calculated estimates of fat, muscle and even bone mass. While it is well-established to provide reliable and valid calculations of fat and muscle mass, its validity for the estimation of bone mass is less certain. While further research is needed in this area, the concept of
Written by Dr Louise Horrigan, Lecturer, University of Galway
In the midst of this quandary, research forges ahead to develop useable and innovative approaches for the improved identification of people at risk of osteoporotic fracture.
being able to quickly provide a full assessment to include an estimate of bone mass, at the same time as the indicators of other conditions associated with body composition, such as obesity, is very appealing. Other point-of-care assessments that can indicate a risk of fracture include functional tests such as handgrip strength, the timed up-and-go test and the one-leg standing test. These functional tests, while often used to assess muscle strength, can also be indicative of bone mass and bone strength, partly due to the close physiological relationship between muscle and bone, but also because they assess aspects of balance and strength, which are critical for the prevention of falls. Indeed, the risk of falling is an aspect of fracture risk that, while influenced by age, bodyweight and other factors, is probably more difficult to quantify than any single physiological or clinical risk factor. Therefore, notwithstanding the importance of good access to DXA, point-of-care assessments such as these have an important complementary role to play in the overall clinical assessment of fracture risk.
So while we await a national screening programme for osteoporosis, researchers and

clinicians will continue to strive in various ways, to innovate, to collaborate, and to develop, in order to shatter the silence on this debilitating and life-limiting epidemic. Part of this work is to increase the utility of point-of-care assessments, in order to improve the identification of people at risk of fracture, and ultimately to reduce the risk and improve the quality of life for our aging population and their families.
References:
E E, Wang T, Yang L, Dempsey M, Brennan A, Yu M, et al. Utility of Osteoporosis Self-Assessment Tool as a Screening Tool for Osteoporosis in Irish Men and Women: Results of the DXAHIP Project. J Clin Densitom. 2021;24(4):516-26.
Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16(1):82.
Horrigan LA, Cooke M, Diskin J, Brennan A, Carey JJ. Could pointof-care bioimpedance analysis be another tool in the prevention of osteoporotic fractures? J Orthop. 2025;64:29-33.
O’Sulivan, D; Carey JJ https:// hospitalprofessionalnews. ie/2022/05/09/why-ireland-wouldbenefit-from-a-national-screeningfor-osteoporosis/ Hospital Professional News Ireland. 2022 May 9: 46 – 48.
Gregori G, Johansson L, Axelsson KF, Jaiswal R, Litsne H, Larsson BAM, et al. The role of different physical function tests for the prediction of fracture risk in older women. J Cachexia Sarcopenia Muscle. 2024;15(4):1511-9.

For this edition of Hospital Professionals News, Epilepsy Ireland Advocacy & Communications Manager, Paddy McGeoghegan updates us on the organisation’s plans for their biggest awareness-raising day of the year….
I am delighted to once again be writing in this edition of Hospital Professional News to update readers on Epilepsy Ireland’s plans for International Epilepsy Day –thank you to the editorial team for the invite to contribute.
As I write this, we are just weeks away from International Epilepsy Day on February 10th. This is our most important day of the year where we seek to increase public understanding and awareness of a condition that affects over 45,000 people in Ireland today.
This year, like previous years and as guided by the people we represent, we are building a new campaign to highlight the importance of knowing how to correctly respond to a seizure and those three important words of TIME, SAFE, STAY.
The new campaign will be featured on traditional media, TV, digital billboards, radio and of course, social media. The campaign will be fronted by our two incredible volunteers, Emma and Gareth, whose images will be used to show that epilepsy and seizure first aid becomes much
Written by Paddy McGeoghegan, Epilepsy Ireland
clearer when you know about TIME, SAFE, STAY! But what exactly do we mean about by these three key words?
TIME - the first thing you should do is TIME the seizure. This is because if a seizure goes over 5 minutes, an ambulance should be called.
SAFE - keep the person SAFE during the seizure. If a person is having a convulsive seizure, cushion their head with something soft, if possible and remove any harmful objects, e.g. furniture from their vicinity. NEVER put anything in a person's mouth or restrain them during a seizure. Be aware that there are also types of seizures where the person does not experience convulsions. Instead, they may “zone out” or stare blankly, become confused or agitated, display behaviours like chewing, smacking their lips,
fiddling with their clothes, or wandering aimlessly. In this type of seizure, the person’s awareness of their surroundings is affected, and it is important to gently guide the person away from any danger. As with convulsive seizures, never restrict the person’s movements.
STAY - during the seizure and after it passes, STAY with the person. Often after a seizure, a person with epilepsy will be confused and, in many cases, exhausted. Make sure to stay with them until recovery is complete, explain what has happened and gently reassure them. The person may have experienced an injury if they have fallen, and if this is the case, ensure normal first aid steps are taken.
This marks the fifth year that we have promoted the key words of TIME, SAFE, STAY when it comes to seizure response, following its inception in 2021. Why do we keep returning to the promotion of these key words? In short, it is working!
For the past 10 years, we have been regularly commissioning and tracking ‘market research’ data on public awareness of and attitudes to epilepsy. It is very interesting and encouraging to look at some of the changes that have taken place in that time:
• A person should be restrained during a seizure - 18% agreed with this statement in 2013 compared to just 5% in 2024.
• Almost all seizures involve falling to the ground followed by stiffening and jerking movements - 43% agreed this statement was true in 2013. This had fallen to 19% in 2024.
• Put an object in a person’s mouth during a seizure - 51% agreed with this statement was true in 2013 vs 21% in 2024.
• Timing the seizure is a key seizure response - 1% said this was correct in 2013 but thanks to TIME-SAFE-STAY, this jumped to 45% by 2024.
Public attitudes do not change overnight and there is still work to do but is clear that we are moving in the right direction – particularly since the introduction of Time, Safe, Stay in 2021.
We hope we will continue to see increased understanding of epilepsy and seizure first aid following February 10th through our new campaign and would appeal to readers of Hospital Professional News to please share TIME, SAFE, STAY with their colleagues, patients, family and friends. We also have detailed posters which can be downloaded, printed and shared from our website.
As always, should Epilepsy Ireland be of assistance to you either in a personal or professional capacity, please do not hesitate to get in touch with us by calling 01 455 7500, emailing info@epilepsy.ie or by visiting www.epilepsy.ie.

RCSI University of Medicine and Health Sciences has welcomed the announcement of Beaumont Hospital’s comprehensive fiveyear strategic plan designed to ensure the highest standards of healthcare delivery and to meet the evolving needs of patients and the community it serves.
The strategy was launched by Minister for Finance Jack Chambers TD, Bernard Gloster HSE CEO and Pauline Philip, Chair of the Beaumont Hospital Board.
Rooted in seven key principles, Building Excellence in Care, Together, is a patient-centred strategy that underscores the hospital’s commitment to excellence, innovation, and sustainability. Beaumont Hospital is one of Ireland’s largest 'model 4' hospitals, employing over 4,935 healthcare professionals, with 851 beds.
Informed by public health, Census 2022 population and hospital inpatient and outpatient data, and insights from stakeholders, the new strategic plan provides a blueprint to ensure Beaumont Hospital can best serve the needs of an aging local population of more than half a million and the complex surgical and advanced care needs of patients across the newly established Dublin North and North East health region.
The plan also addresses the needs of patients across Ireland who require access to national cancer, transplant and neuroscience services, including neurosurgery, neurology, stroke, cochlear implantation and neuro-ICU.
Speaking at the launch, Minister for Finance Jack Chambers TD said, “As Government investment
Minister for Finance
Jack Chambers TD
into the health sector has dramatically increased in recent years, it is important that Ireland’s healthcare facilities like Beaumont Hospital develop and implement strategies such as the one launched here today. This strategy will ensure the delivery of a truly integrated care system across a range of multidisciplinary services at one of Ireland’s largest 'model 4' hospitals, while also helping to provide urgent and emergency care for the community which Beaumont Hospital serves. It is the development and implementation of strategies like this that help to increase productivity, advance Irish healthcare, and will secure further investment into our patientcentred services.”
Welcoming the launch, Professor Cathal Kelly, Vice Chancellor of RCSI, said, “RCSI is proud to be the primary academic education and research partner to Beaumont Hospital. We have worked closely with the Hospital since it’s foundation almost 40 years ago, and together we have been at the forefront of training future generations of healthcare professionals and collaborating on highly impactful research to improve patient care. We look forward to this continued partnership with Beaumont on their strategic journey over the next five years and realising our shared ambitions for excellence in education and research for the benefit of human health.”


are:
1. Patients
Our patients remain at the heart of everything we do. The hospital is committed to delivering patient-centred care that is safe, compassionate, and equitable. Enhancing the patient experience will continue to drive all service improvements and initiatives.
2. Our people and culture
Beaumont Hospital recognises that its greatest asset is its workforce. By fostering a culture of inclusivity, respect, and professional growth, the hospital aims to attract, retain and empower its staff to excel in their roles and deliver world-class care.
3. Our clinical and corporate governance
Strong governance ensures accountability and continuous improvement. Beaumont Hospital will enhance its clinical and corporate structures to maintain the highest standards of safety, transparency, and organisational integrity.
4. Our physical, diagnostic and digital infrastructure
Investing in state-of-the-art infrastructure is critical to delivering cutting-edge care. The hospital will prioritise modernisation of facilities,
adoption of advanced diagnostic tools, and the integration of digital health technologies to improve accessibility and efficiency.
5. Our national, regional and local service profile
As a leading healthcare provider, Beaumont Hospital is committed to strengthening its role and further developing its profile across all levels of the health system. Collaboration with national, regional, and local partners will ensure that services are aligned with community needs and healthcare priorities.
6. Our clinical, educational and research partnerships
Partnerships with academic institutions and research organisations are vital to innovation and excellence. Beaumont Hospital will expand its collaborations to drive advancements in clinical practice, education, and medical research.
7. Our performance, productivity and sustainability
To ensure a resilient future, Beaumont Hospital will focus on enhancing operational efficiency, financial sustainability, and environmental stewardship. Performance metrics will guide continuous improvement and accountability across all areas. Values guiding the plan
HSE CEO Bernard Gloster
The strategic plan is underpinned by Beaumont Hospital’s core values of Excellence, Compassion, Respect, and Patient Focused Quality Care. These principles ensure that the hospital remains steadfast in its mission to deliver exceptional care and inspire trust within the community.
Treatment of Acute Coronary Syndrome1
2.5 mg
Product subject to medical prescription which may not be renewed (A)

Reference: 1. Arixtra® 2.5 mg/0.5 ml solution for injection, pre-filled syringe. Summary of Product Characteristics (SmPC). Available at: www.medicines.ie. Last accessed: August 2024.
ABBREVIATED PRESCRIBING INFORMATION
Arixtra (fondaparinux sodium) 2.5 mg/0.5 ml solution for injection, pre-filled syringe
Please refer to Summary of Product Characteristics (SmPC) before prescribing Indications, Dosage and Administration:
Indications:
Prevention of Venous Thromboembolic Events (VTE) in adults undergoing major orthopaedic surgery of the lower limbs such as hip fracture, major knee surgery or hip replacement surgery. Prevention of Venous Thromboembolic Events (VTE) in adults undergoing abdominal surgery who are judged to be at high risk of thromboembolic complications, such as patients undergoing abdominal cancer surgery. Prevention of Venous Thromboembolic Events (VTE) in adult medical patients who are judged to be at high risk for VTE and who are immobilised due to acute illness such as cardiac insu ciency and/or acute respiratory disorders, and/or acute infectious or inflammatory disease.
Treatment of unstable angina or non-ST segment elevation myocardial infarction (UA/NSTEMI) in adults for whom urgent (<120 mins) invasive management (PCI) is not indicated. Treatment of ST segment elevation myocardial infarction (STEMI) in adults who are managed with thrombolytics or who initially are to receive no other form of reperfusion therapy. Treatment of adults with acute symptomatic spontaneous superficial-vein thrombosis of the lower limbs without concomitant deep-vein thrombosis.
Dosage
Patients undergoing major orthopaedic or abdominal surgery: The recommended dose of fondaparinux is 2.5 mg once daily administered post-operatively by subcutaneous injection. The initial dose should be given 6 hours following surgical closure provided that haemostasis has been established. Treatment should be continued until the risk of venous thromboembolism has diminished, usually until the patient is ambulant, at least 5 to 9 days after surgery.
Medical patients who are at high risk for thromboembolic complications based on an individual risk assessment: The recommended dose of fondaparinux is 2.5 mg once daily administered by subcutaneous injection. A treatment duration of 6-14 days has been clinically studied in medical patients.
Treatment of unstable angina/non-ST segment elevation myocardial infarction (UA/NSTEMI): The recommended dose of fondaparinux is 2.5 mg once daily, administered by subcutaneous injection. Treatment should be initiated as soon as possible following diagnosis and continued for up to a maximum of 8 days or until hospital discharge if that occurs earlier. If a patient is to undergo percutaneous coronary intervention (PCI), unfractionated heparin (UFH) as per standard practice should be administered during PCI, taking into account the
patient’s potential risk of bleeding, including the time since the last dose of fondaparinux, the timing of restarting subcutaneous fondaparinux after sheath removal should be based on clinical judgment. In the pivotal UA/NSTEMI clinical trial, treatment with fondaparinux was restarted no earlier than 2 hours after sheath removal.
Treatment of ST segment elevation myocardial infarction (STEMI): The recommended dose of fondaparinux is 2.5 mg once daily. The first dose of fondaparinux is administered intravenously and subsequent doses are administered by subcutaneous injection. Treatment should be initiated as soon as possible following diagnosis and continued for up to a maximum of 8 days or until hospital discharge if that occurs earlier. If a patient is to undergo non-primary PCI, unfractionated heparin (UFH) as per standard practice should be administered during PCI, taking into account the patient’s potential risk of bleeding, including the time since the last dose of fondaparinux. The timing of restarting subcutaneous fondaparinux after sheath removal should be based on clinical judgment. In the pivotal STEMI clinical trial, treatment with fondaparinux was restarted no earlier than 3 hours after sheath removal.
Patients who are to undergo coronary artery bypass graft (CABG) surgery: In STEMI or UA/NSTEMI patients who are to undergo coronary artery bypass graft (CABG) surgery, fondaparinux where possible, should not be given during the 24 hours before surgery and may be restarted 48 hours post-operatively.
Treatment of superficial-vein thrombosis: The recommended dose of fondaparinux is 2.5 mg once daily, administered by subcutaneous injection. Patients eligible for fondaparinux 2.5 mg treatment should have acute, symptomatic, isolated, spontaneous superficial-vein thrombosis of the lower limbs, at least 5 cm long and documented by ultrasonographic investigation or other objective methods. Treatment should be initiated as soon as possible following diagnosis and after exclusion of concomitant DVT or superficial-vein thrombosis within 3 cm from the sapheno-femoral junction. Treatment should be continued for a minimum of 30 days and up to a maximum of 45 days in patients at high risk of thromboembolic complications. Patients could be recommended to self-inject the product when they are judged willing and able to do so. Physicians should provide clear instructions for self-injection. Patients who are to undergo surgery or other invasive procedures: In superficial vein thrombosis patients who are to undergo surgery or other invasive procedures, fondaparinux, where possible, should not be given during the 24 hours before surgery. Fondaparinux may be restarted at least 6 hours post-operatively provided haemostasis has been achieved.
Special populations: Prevention of VTE following Surgery: In patients undergoing surgery, timing of the first fondaparinux injection requires strict adherence in patients ≥75 years, and/or with body weight <50 kg and/or with renal impairment with creatinine clearance ranging between 20 to 50 ml/min. The first fondaparinux administration should be given not earlier than 6 hours following surgical closure. The injection should not be given unless haemostasis has been established.

Renal impairment:
Prophylaxis of VTE - Fondaparinux should not be used in patients with creatinine clearance <20 ml/min. The dose should be reduced to 1.5 mg once daily in patients with creatinine clearance in the range of 20 to 50 ml/min. No dosage reduction is required for patients with mild renal impairment (creatinine clearance >50 ml/min). Treatment of UA/NSTEMI and STEMI - Fondaparinux should not be used in patients with creatinine clearance <20 ml/min. No dosage reduction is required for patients with creatinine clearance >20 ml/min.
Treatment of superficial-vein thrombosis - Fondaparinux should not be used in patients with creatinine clearance <20 ml/min. The dose should be reduced to 1.5 mg once daily in patients with creatinine clearance in the range of 20 to 50 ml/min. No dosage reduction is required for patients with mild renal impairment (creatinine clearance >50 ml/min).
Hepatic impairment:
Prevention of VTE and Treatment of UA/NSTEMI and STEMI - No dosing adjustment is necessary in patients with either mild or moderate hepatic impairment. In patients with severe hepatic impairment, fondaparinux should be used with care as this patient group has not been studied. Treatment of superficial-vein thrombosis - The safety and e cacy of fondaparinux in patients with severe hepatic impairment has not been studied, therefore fondaparinux is not recommended for use in these patients.
Paediatric population - Fondaparinux is not recommended for use in children below 17 years of age due to a lack of data on safety and e cacy.
Low body weight: Prevention of VTE and Treatment of UA/NSTEMI and STEMIPatients with body weight <50 kg are at increased risk of bleeding. Elimination of fondaparinux decreases with weight. Fondaparinux should be used with caution in these patients. Treatment of superficial-vein thrombosis - The safety and e cacy of fondaparinux in patients with body weight less than 50 kg has not been studied, therefore fondaparinux is not recommended for use in these patients.
Administration
Subcutaneous administration: Fondaparinux is administered by deep subcutaneous injection while the patient is lying down. Sites of administration should alternate between the left and the right anterolateral and left and right posterolateral abdominal wall. To avoid the loss of medicinal product when using the pre-filled syringe do not expel the air bubble from the syringe before the injection. The whole length of the needle should be inserted perpendicularly into a skin fold held between the thumb and the forefinger; the skin fold should be held throughout the injection.
Intravenous administration (first dose in patients with STEMI only) Intravenous administration should be through an existing intravenous line either directly or using a small volume (25 or 50 ml) 0.9% saline minibag. To avoid the loss of medicinal product when using the pre-filled syringe do not expel the air bubble from the syringe before the injection. The intravenous tubing should be well flushed with saline after injection to ensure that all of the medicinal product is administered. If administered via a minibag, the infusion should be given over 1 to 2 minutes.
Presentation: Solution for injection
Contraindications:
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Active clinically significant bleeding.
Acute bacterial endocarditis.
Severe renal impairment defined by creatinine clearance < 20 ml/min.
Warnings and precautions: Fondaparinux is intended for subcutaneous use only. Do not administer intramuscularly.
There is limited experience from treatment with fondaparinux in haemodynamically unstable patients and no experience in patients requiring thrombolysis, embolectomy or insertion of a vena cava filter.
Haemorrhage: Fondaparinux should be used with caution in patients who have an increased risk of haemorrhage, such as those with congenital or acquired bleeding disorders (e.g. platelet count <50,000/mm3), active ulcerative gastrointestinal disease and recent intracranial haemorrhage or shortly after brain, spinal or ophthalmic surgery and in special patient groups. As for other anticoagulants, fondaparinux should be used with caution in patients who have undergone recent surgery (<3 days) and only once surgical haemostasis has been established.
(For prevention of VTE) Agents that may enhance the risk of haemorrhage should not be administered concomitantly with fondaparinux. These agents include desirudin, fibrinolytic agents, GP IIb/IIIa receptor antagonists, heparin, heparinoids, or Low Molecular Weight Heparin (LMWH). When required, concomitant therapy with vitamin K antagonist should be administered. Other antiplatelet medicinal products (acetylsalicylic acid, dipyridamole, sulfinpyrazone, ticlopidine or clopidogrel), and NSAIDs should be used with caution. If co-administration is essential, close monitoring is necessary.
For treatment of UA/NSTEMI, STEMI and superficial-vein thrombosis: Fondaparinux should be used with caution in patients who are being treated concomitantly with other agents that increase the risk of haemorrhage (such as GPIIb/IIIa inhibitors or thrombolytics).
PCI and risk of guiding catheter thrombus: In STEMI patients undergoing primary PCI, the use of fondaparinux prior to and during PCI is not recommended. Similarly, in UA/NSTEMI patients with life threatening conditions that require urgent revascularisation, the use of fondaparinux prior to and during PCI is not recommended. These are patients with refractory or recurrent angina associated with dynamic ST deviation, heart failure, life-threatening arrhythmias or haemodynamic instability.
In UA/NSTEMI and STEMI patients undergoing non-primary PCI: the use of fondaparinux as the sole anticoagulant during PCI is not recommended due to an increased risk of guiding catheter thrombus. Therefore adjunctive UFH should be used during non-primary PCI according to standard practice.
Patients with superficial-vein thrombosis: Presence of superficial-vein thrombosis greater than 3 cm from the sapheno-femoral junction should be confirmed and concomitant DVT should be excluded by compression ultrasound or objective methods prior to initiating treatment of fondaparinux.
Spinal/Epidural anaesthesia:
In patients undergoing major orthopaedic surgery, epidural or spinal haematomas that may result in long-term or permanent paralysis cannot be excluded with the concurrent use of fondaparinux and spinal/epidural
anaesthesia or spinal puncture. The risk of these rare events may be higher with post-operative use of indwelling epidural catheters or the concomitant use of other medicinal products a ecting haemostasis.
Elderly patients: The elderly population is at increased risk of bleeding. As renal function is generally decreasing with age, elderly patients may show reduced elimination and increased exposure of fondaparinux. Fondaparinux should be used with caution in elderly patients.
Low body weight: Prevention of VTE and Treatment of UA/NSTEMI and STEMI - Patients with body weight <50 kg are at increased risk of bleeding. Fondaparinux should be used with caution at a daily dose of 5 mg in this population. Elimination of fondaparinux decreases with weight. Fondaparinux should be used with caution in these patients.
Treatment of superficial-vein thrombosis - Fondaparinux is not recommended for treatment of superficial-vein thrombosis in patients with body weight less than 50 kg.
Renal impairment: Fondaparinux is known to be mainly excreted by the kidney. Prophylaxis of VTE - Patients with creatinine clearance <50 ml/min are at increased risk of bleeding and VTE and should be treated with caution. For the treatment of UA/NSTEMI and STEMI- there are limited clinical data available on the use of fondaparinux 2.5 mg once daily in patients with creatinine between 20 and 30 ml/min. Therefore, the physician should determine if the benefit of treatment outweighs the risk. For treatment of superficial-vein thrombosis - Fondaparinux should not be used in patients with creatinine clearance <20 ml/min. The dose should be reduced to 1.5 mg once daily in patients with creatinine clearance in the range of 20 to 50 ml/min.
Severe hepatic impairment: Prevention of VTE and Treatment of UA/NSTEMI and STEMI - Dosing adjustment of fondaparinux is not necessary. However, the use of fondaparinux should be considered with caution because of an increased risk of bleeding due to a deficiency of coagulation factors in patients with severe hepatic impairment.
Treatment of superficial-vein thrombosis - Fondaparinux is not recommended for the treatment of superficial-vein thrombosis in these patients.
Heparin Induced Thrombocytopenia: Fondaparinux should be used with caution in patients with a history of HIT.
Latex Allergy: The needle shield of the pre-filled syringe may contain dry natural latex rubber that has the potential to cause allergic reactions in latex sensitive individuals.
Interactions with other medicinal products and other forms of interaction: Bleeding risk is increased with concomitant administration of fondaparinux and agents that may enhance the risk of haemorrhage. In clinical studies performed with fondaparinux, oral anticoagulants (warfarin) did not interact with the pharmacokinetics of fondaparinux; at the 10 mg dose used in the interaction studies, fondaparinux did not influence the anticoagulation monitoring (INR) activity of warfarin. Platelet inhibitors (acetylsalicylic acid), NSAIDs (piroxicam) and digoxin did not interact with the pharmacokinetics of fondaparinux. At the 10 mg dose used in the interaction studies, fondaparinux did not influence the bleeding time under acetylsalicylic acid or piroxicam treatment, nor the pharmacokinetics of digoxin at steady state.
If follow-up treatment is to be initiated with heparin or LMWH, the first injection should, as a general rule, be given one day after the last fondaparinux injection. If follow up treatment with a Vitamin K antagonist is required, treatment with fondaparinux should be continued until the target INR value has been reached.
Fertility, pregnancy and lactation:
Pregnancy: No clinical data on exposed pregnancies are available. Animal studies are insu cient with respect to e ects on pregnancy, embryo/foetal development, parturition and postnatal development because of limited exposure. Fondaparinux should not be prescribed to pregnant women unless clearly necessary.
Breast-feeding: Fondaparinux is excreted in rat milk but it is not known whether fondaparinux is excreted in human milk. Breast-feeding is not recommended during treatment with fondaparinux. Oral absorption by the child is however unlikely.
Fertility: There are no data available on the e ect of fondaparinux on human fertility. Animal studies do not show any e ect on fertility.
Undesirable effects:
Serious adverse reactions reported with fondaparinux are bleeding complications (various sites including rare cases of intracranial/intracerebral and retroperitoneal bleedings). Fondaparinux should be used with caution in patients who have an increased risk of haemorrhage.
Very common (≥1/10): None. Common (≥1/100, <1/10): anaemia, post-operative haemorrhage, uterovaginal haemorrhage, haemoptysis, haematuria, haematoma, gingival bleeding, purpura, epistaxis, gastrointestinal bleeding, hemarthrosis, ocular bleeding, bruise.
For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC.
Reporting of adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse reactions/events should also be reported to the marketing authorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267.
Legal Category: Product subject to prescription which may not be renewed (A).
Marketing Authorisation Number: EU/1/02/206/003
Marketing Authorisation Holder: Viatris Healthcare Limited, Damastown Industrial Park, Mulhuddart, Dublin 15, DUBLIN, Ireland
Full prescribing information available on request from: Viatris, Dublin 17. Email: enquiry.ire@viatris.com
Date of Revision of Abbreviated Prescribing Information: 27 Feb 2024
Reference Number: IE--AbPI-Arixtra-2.5mg-v003
Authors Cara M Martin
and John J O’Leary on behalf of the CERVIVA consortium
Cara Martin BSc (Hons), MSc, PhD, FRCPath, Associate Professor in Molecular Pathology, Tumour Biology and Cancer Screening, Cancer Prevention Research Theme Co-Lead, Trinity St. James's Cancer Institute, Co-Lead of CERVIVA Research Consortium, School of Medicine, Trinity College Dublin.
John O’Leary MD, DSc, PhD (Oxon), MSc, MA (j.o.), MA (Oxon), FRCPath, FFPathRCPI, FRCPI, FTCD. Chair of Pathology, Trinity College Dublin - Co-Lead of CERVIVA Research Consortium, Discipline of Histopathology and Morbid Anatomy, The School of Medicine, Trinity College Dublin.


John J O’Leary
Cervical cancer is a largely preventable disease but worldwide it remains one of the most common cancers and causes of cancer-related death in women. In 2020, the World Health Organization launched the global strategy to accelerate the elimination of cervical cancer as a public health problem.1 Elimination of cervical cancer as a public health problem will be reached by achieving fewer than four new cases per 100,000 women in every country. To achieve this goal, three targets have been clearly identified to accelerate elimination and achieve this goal.
• 90% of girls to be fully vaccinated with the Human Papilloma Virus (HPV) vaccine by age 15,
• 70% of women to be screened by age 35 and again by age 45 using a high-performance test (equivalent to or better than an HPV test),
• 90% of women identified with cervical disease (precancerous lesions or invasive cancer) to receive treatment.
In Ireland, the current cervical cancer rate is estimated at 10.1 per 100,000 women,2 with almost 290 new cases being diagnosed
annually. Ireland is in an excellent position to move rapidly towards cervical cancer elimination, having access to excellent populationbased cervical screening and HPV vaccination programmes. Indeed, the HSE have recently published a roadmap and action plan to align with the WHO targets setting 2040 as a target date for Ireland to achieve cervical cancer elimination.2 Since the introduction of CervicalCheck The National Cervical Screening Programme in 2008,3 Ireland has seen a year on year 2.8% decline in the incidence of cervical cancer. Furthermore, following the introduction of HPV vaccination in 2010, some early data is emerging on the positive impact of HPV vaccination in reducing the number of women presenting with abnormal cervical screening tests.4
Virtually, all cases of cervical cancer are caused by HPV, however, HPV infection also increases the risk of other cancers such as vulval, vaginal, penile, anal and head and neck cancer. Ongoing research in Ireland suggests there remains a lack of awareness of the role HPV plays in these other cancers. Moreover, the incidence of most of these other HPV associated cancers is increasing in Ireland.5 HPV
vaccination reduces the risk of these cancers too, and we must do more to increase uptake of the HPV vaccine among girls and boys, by increasing awareness of the role of HPV in these other cancer types and the benefits of HPV vaccination for the prevention of these cancers in both men and women.
Our research group CERVIVA is a multi-investigator consortium led by Professor John O’Leary and Professor Cara Martin of Trinity College Dublin, encompassing researchers at several national and international academic institutions, and health agencies (www.cerviva.ie). The consortium was established in 2005, with the purpose to advance high quality peer-reviewed research programmes in HPV associated diseases and it continues to conduct transformative research in this area.
Our research programme is focussed developing new improved cervical screening approaches, for both vaccinated and unvaccinated populations, understanding barriers to screening and vaccinations among certain groups and developing new screening approaches and biomarkers for early detection of other HPV associated cancers including vulva, vagina cancers, anal cancer, and oral cancers. Some of our flagship research programmes that support the HSE’s plan for cervical cancer elimination include the following:
CERVIVA HPV Primary Screening Study
Molecular triage strategies for HPV positive women, funded by the Health Research Board. This is an observational cohort study which has recruited over 13,000 women (aged 25-60years) who attended primary care for their routine cervical smear. The study
led by TCD was established in partnership with CervicalCheck the National Cervical Screening Programme in Ireland, to evaluate a range of different HPV and novel biomarker tests and technologies for use in cervical screening, specifically in the context of HPVbased primary screening. Our study assesses a range of different biomarker and triage options for use with HPV based screening to help stratify those women at risk of developing cervical cancer and show the potential benefits of including additional biomarkers such as for example partial HPV genotyping, methylation biomarkers and p16ink4a/ki67 biomarkers into the screening algorithm.6, 7 The study is the first of its kind internationally that is looking at this range of molecular triage tests and their performance longitudinally. The study is ongoing and will continue to collect data for 10 years following participants initial enrolment in the study.
CERVIVA-Vax: Monitoring the impact of HPV vaccination in Ireland, funded by the Health Research Board
The introduction of HPV vaccination and changes to cervical screening protocols with introduction of HPV testing, raise important clinical challenges for cervical screening programmes, most notably regarding the impact of HPV vaccination on screening as it is currently organised and how this will affect the disease landscape and sub-type of human papilloma virus (HPV), present in the Irish population. Monitoring the impact of vaccination on screening is hugely important.
CERVIVA-Vax will generate Irish data relating to the early impact of HPV vaccination on cervical screening. By investigating the early impact of HPV vaccination on screening in Ireland, CERVIVAVax will be able to inform policy
and practice in relation to the best cervical screening approaches for vaccinated and unvaccinated women. Indeed, together with the National Screening Service, some early evidence is available on the impact of HPV vaccination at reducing the number of women presenting with abnormal cervical screening tests.4
What influences cervical screening uptake in older women and how can screening programmes translate this knowledge into behaviour changing strategies? A CERVIVA-Cervical Check coproduction project
Well-organised cervical screening is effective in reducing cervical cancer incidence and mortality. To achieve these benefits, high coverage is essential. In Ireland, the coverage target is 80%.2 While overall coverage has risen from 74.7% in the first 5 years to 78.3% in the 5 years to 2020, it has consistently been lower in older (50-60years) than younger (25-49years) women.8 This distinctive pattern is not seen in other countries with organised programmes and the reasons for it are unknown. This CERVIVA CervicalCheck co-production
project is generating evidence on the influences on cervical screening participation among older women in Ireland, to inform development and implementation of evidence-based strategies to increase screening coverage in this group. We are learning more about the barrier’s women in this group face with respect to participation in screening, ranging from previous experiences to ease of arranging appointments for example.
On this Cervical Cancer Prevention Week, we continue to work towards the vision of an Ireland where cervical cancer and indeed all HPV associated cancers are rare in every community. Our research programme supports the National Action Plan for Cervical Cancer Elimination. We encourage all eligible woman to avail of cervical screening and encourage all parents to vaccinate their children are to ensure they are protected against HPV.
References:
1. https://www.who.int/initiatives/ cervical-cancer-eliminationinitiative
2. Ireland’s Cervical Cancer Elimination Action Plan
2025-2030. Strategic Vision 2025-2040 https://assets.hse. ie/media/documents/Irelands_ Cervical_Cancer_Elimination_ Action_Plan_2025-2030.pdf
3. National Cancer Registry Ireland. Cancer Trends No. 38. Breast, cervical and colorectal cancer 1994-2019: National trends for cancers with population-based screening programmes in Ireland. NCRI; 2022. Available from. https:// www.ncri.ie/sites/ncri/files/pubs/ Trendsreport_breast_cervical_ colorectal_22092022.pdf
4. Rourke, M., Fitzpatrick, P., Popoola, O., Boms, R., Mooney, T., Heavey, L., Mason Mohan, C., Martin, C.M., Jessop, L, Russell, N.E. The effect of HPV vaccination on the rate of high-grade cytology in 25-year-old women attending cervical screening in Ireland. Ir J Med Sci 193, 665–668 (2024). https://doi.org/10.1007/s11845023-03551-y
5. National Cancer Registry Ireland. Cancer Trends No 40. HPV-associated cancers 2024. Available from https://www.ncri. ie/sites/ncri/files/pubs/NCRI_ HPVRelatedCancers_2024_0.pdf
6. White, C, Reynolds, S, Murphy, K, Keegan, H, Naik, P, O'Brien, R, Pilkington, L, Sharkey Ochoa, I Gleeson, G, Russell, N, Nuttall, D, Tewari, P, Wright, F O'Toole, S, Sharp, L, Flannelly, G, O'Leary, JJ, Martin, CM. Performance of the HPV E6/E7 mRNA Aptima HPV assay combined with partial genotyping compared with the HPV DNA Cobas 4800 HPV test for use in primary screening: Results from the CERVIVA HPV Primary Screening Study in Ireland. Int J Cancer. 2023 Aug 26. doi: 10.1002/ijc.34685. Online ahead of print. PMID: 37632406
7. O'Leary JJ, White C, Spillane C, Naik, P, O'Brien, R, Reynolds, S, Pham, T, Pilkington, L, Sharkey Ochoa, I, Bolger, N, Barry O'Crowley, J, Tewari, P, O'Toole, S, Sweeney, M, Keegan, H, Normand, C, Sharp, L, Flannelly, G, Martin, CM. Cervical screening: A new way forward (tests of risk and tests of disease). HRB Open Res 2018, 1:3 (doi: 10.12688/ hrbopenres.12794.1)
8. CervicalCheck Programme Report September 2017-March 2020
Written by Dr S Griffiths, Dr D Breen, Department of Respiratory Medicine, Galway University Hospital
Abstract: Smoking remains the single leading cause of preventable disease, disability and death worldwide. Currently 18% of Irish adults are active smokers, and recent years have seen an upward trend of young people developing a nicotine addiction, in part due to the rise in popularity of electronic nicotine delivery systems (ENDS). Smoking cessation services are an essential component of tobacco control, and play a key role in meeting the goal of a Tobacco-Free Ireland.
Introduction:
In this paper we will analyse current prevalence and trends in smoking habits in Ireland, and discuss the impact of ENDS in the market, particularly the impact on Irish teenagers and potential legislation to tackle this novel challenge. We aim to identify successful smoking cessation frameworks worldwide and discuss the benefits to both the patient and the health system. A local audit conducted in Galway University Hospital provides insight into current smoking cessation practise and we have identified potential methods to improve local systems. We will review the National Stop Smoking Guideline 2022, in particular it’s application to the hospital setting and discuss the need for increased training among all healthcare staff in smoking cessation provision.
Meeting the goal of being tobacco-free by 2025 is dependent on accelerating progress with smoking cessation in Ireland, and this paper aims to identify current challenges to Irish smoking habits and identify an effective approach to improving our smoking cessation services.
Tobacco use is the leading cause of preventable death, disease and disability worldwide. (1) The Organisation (WHO) describes current tobacco usage worldwide as a global epidemic. The Healthy Report 2022 (HI) reports that 18% of the population of Ireland are current smokers. There has significant shift in demographics in Ireland with the Central Statistics Office publishing data for 2022, showing the largest 12 -month increase in the population of Ireland since 2008, in part accounted 15-year high in immigrant arrivals (2). These demographic shifts will have a major impact on smoking prevalence; for example the rapid increase in Ukrainian nationals to Ireland will ultimately resul in overall smoking numbers; it was estimated that in 2022, 27.4% of the Ukrainian adult population (3).
The HI survey also reflects WHO data on long term health effects of tobacco use, reporting that 32% of smokers and 39% of ex-smokers suffer from longterm illness and chronic health problems, compared with 27% of never smokers.4 Year on year, the HI Survey identifies 25–34-yearolds as the age group most likely to smoke, and the proportion of smokers in this group has experienced a 4-point increase to 24%, significantly higher than the national average.4 Smoking prevalence by age and gender is illustrated in Figure 1 and of note highlights the persistent high smoking rates within young adults, particularly males, and the decrease in smoking prevalence among older adults.
Current prevalence and trends in smoking habits:
Tobacco use is the leading cause of preventable death, disease and disability worldwide.1 The World Health Organisation (WHO) describes current tobacco usage worldwide as a global epidemic. The Healthy Ireland Report 2022 (HI) reports that 18% of the population of Ireland are current smokers. There has been a significant shift in demographics in Ireland with the Central Statistics Office publishing data for the year ending 2022, showing the largest 12-month increase in the population of Ireland since 2008, in part accounted for by a 15year high in immigrant arrivals.2 These demographic shifts will have a major impact on smoking prevalence; for example the rapid increase in Ukrainian nationals to Ireland will ultimately result in an increase in overall smoking numbers; it was estimated that in 2022, 27.4% of the Ukrainian adult population smoked.3
The European Schools Project on Alcohol and other Drugs (ESPAD) Ireland 2019 Report5 provides information about substance use among Irish teenagers attending secondary school, and reported that the decline in smoking has halted in Irish teenagers for the first time in 25 years. This correlated with a higher prevalence of e-cigarette use among Irish teenagers than smoking tobacco, with 39% of 15- to 16-year-olds reporting having used ENDS, compared with 32% smoking tobacco. There are concerns about the potential role of ENDS providing new routes into nicotine addiction, with 68% of adolescents in a 2019 study reporting they had never used tobacco prior to first use of e-cigarettes.6
The HI survey also reflects WHO data on long term health effects of tobacco use, reporting that and 39% of ex-smokers suffer from long-term illness and chronic health problems, compared with smokers. (4 ) Year on year, the HI Survey identifies 25 –34-year-olds as the age group most likely the proportion of smokers in this group has experienced a 4 -point increase to 24%, significantly national average. (4 ) Smoking prevalence by age and gender is illustrated in Figure 1 and of note persistent high smoking rates within young adults, particularly males, and the decrease in smoking among older adults.
The use of electronic nicotine delivery systems (ENDS), often referred to as e-cigarettes or
‘vapes’ is increasingly prevalent, with a major expansion of this market in Ireland over the past two decades. Overall, 6% of the population reported using ENDS regularly, with the highest use reported in those under the age of 25 (11%).4 ENDS are highly variable, without a strict regulatory framework, and at present there is no definitive research on the long-term impacts of ENDS use, or second-hand exposure. As the popularity of ENDS grows, there is concern about their use in younger people. The Public Health (Tobacco Products and Nicotine Inhaling Products) Bill 2023 is currently before Dáil Éireann, and proposes to introduce a regulatory framework for the retail sale of ENDS, termed ‘nicotine inhaling products’. These measures focus on reducing access to ENDS for children by restricting the sale of these products, and plans to prohibit advertisement of ENDS around schools and on public transport

Film-coated Tablets
varenicline
Available on private prescription only.

Indications
Varenicline Teva 0.5 mg and Varenicline Teva 1 mg Film-coated Tablets (initiation pack) and Varenicline Teva 1 mg
Film-coated Tablets
Varenicline Teva is indicated for smoking cessation in adults.
Varenilcine 0.5mg and 1mg Film-Coated Tablets Abbreviated Prescribing Information Presentation: Each film-coated tablet contains varenicline citrate equivalent to 0.5mg and 1mg varenicline. Indications: Varenicline is indicated for smoking cessation in adults. Dosage and administration: Oral use. Adults: The recommended dose is 1mg Varenicline twice daily following a 1-week titration (see SmPC for details). Children: Not recommended for use. Elderly: No dosage adjustment is necessary. Elderly patients are more likely to have decreased renal function, prescribers should consider the renal status of an elderly patient. Renal impairment: No dosage adjustment is necessary for patients with mild (estimated creatinine clearance >50ml/min and ≤80ml/min) to moderate (estimated creatinine clearance ≥30ml/min and ≤50ml/min) renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30ml/min), the recommended dose of Varenicline is 1mg once daily. Hepatic impairment: No dosage adjustment is necessary. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: Physiological changes resulting from smoking cessation, with or without treatment with Varenicline, may alter the pharmacokinetics or pharmacodynamics of some medicinal products, for which dosage adjustment may be necessary (examples include theophylline, warfarin and insulin). As smoking induces CYP1A2, smoking cessation may result in an increase of plasma levels of CYP1A2 substrates. Changes in behaviour or thinking, anxiety, psychosis, mood swings, aggressive behaviour, depression, suicidal ideation and behaviour and suicide attempts have been reported in patients attempting to quit smoking with Varenicline. Depressed mood, rarely including suicidal ideation and suicide attempt, may be a symptom of nicotine withdrawal. Clinicians should be aware of the possible emergence of serious neuropsychiatric symptoms in patients attempting to quit smoking with or without treatment. If serious
neuropsychiatric symptoms occur whilst on Varenicline treatment, patients should discontinue Varenicline immediately and contact a healthcare professional for reevaluation of treatment. Smoking cessation, with or without pharmacotherapy, has been associated with exacerbation of underlying psychiatric illness (e.g. depression). In clinical trials and post-marketing experience there have been reports of seizures in patients with or without a history of seizures, treated with Varenicline. Varenicline should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold. At the end of treatment, discontinuation of Varenicline was associated with an increase in irritability, urge to smoke, depression, and/or insomnia in up to 3% of patients. In such instances, tapering should be considered. Patients taking Varenicline should seek immediate medical attention if they experience signs and symptoms of myocardial infarction or stroke. Interactions: Varenicline has no clinically meaningful drug interactions (see SmPC for further details). No dosage adjustment of Varenicline or co-administered medicinal products listed below is recommended. In vitro studies indicate that Varenicline is unlikely to alter the pharmacokinetics of compounds that are primarily metabolised by cytochrome P450 enzymes. Furthermore, since metabolism of Varenicline represents less than 10% of its clearance, active substances known to affect the cytochrome P450 system are unlikely to alter the pharmacokinetics of Varenicline, therefore a dose adjustment of Varenicline would not be required. Varenilcine is not known to affect the pharmacokinetics of metformin, digoxin, bupropion and warfarin. Co-administration of cimetidine, with Varenicline increased the systemic exposure of varenicline by due to a reduction in varenicline renal clearance. In patients with severe renal impairment, the concomitant use of cimetidine and Varenicline should be avoided. Pregnancy and lactation: As a precautionary
measure, it is preferable to avoid the use of varenicline during pregnancy. A decision on whether to continue/ discontinue breast-feeding or to continue/discontinue therapy with varenicline should be made taking into account the benefit of breast-feeding to the child and the benefit of varenicline therapy to the woman. Effects on ability to drive and use machines: Varenicline may have minor or moderate influence on the ability to drive and use machines. Varenicline may cause dizziness, somnolence and transient loss of consciousness, and therefore may influence the ability to drive and use machines. Adverse reactions: Diabetes mellitus, suicidal ideation, depression, hallucinations, psychosis, seizure, cerebrovascular accident, transient loss of consciousness, myocardial infarction, angina pectoris, tachycardia, atrial fibrillation, electrocardiogram ST segment depression, gastritis, haematemesis, severe cutaneous reactions including Stevens Johnson Syndrome and Erythema Multiforme, angioedema. Very Common: Nasopharyngitis, abnormal dreams, insomnia, headache, nausea. Common: Bronchitis, sinusitis, weight increased, decreased appetite, increased appetite, somnolence, dizziness, dysgeusia, dyspnoea, cough, gastrooesophageal reflux disease, vomiting, constipation, diarrhoea, abdominal distension, abdominal pain, toothache, dyspepsia, flatulence, dry mouth, rash, pruritus, arthralgia, myalgia, back pain, chest pain, fatigue, liver function test abnormal. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: In case of overdose, standard supportive measures should be instituted as required. Legal category: POM. Marketing Authorisation Number: 0.5mg PA1986/129/001, 1mg PA1986/129/002. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00084. Date of Preparation: July 2024.
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie Prescription Only Medicine.
Further information is available on request or in
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
In the adolescent population, e-cigarettes are rarely cited as a smoking cessation technique, with only 3% of adolescents reporting quitting smoking as the reason for first using e-cigarettes.6 This contrasts with the high ENDS use as a smoking cessation tool in adults; the HSE National Stop Smoking Guideline reports 38% of people choosing e-cigarettes as a quit smoking aid in 2019.7
Tackling tobacco use
Tackling tobacco use has become a priority for government policy makers worldwide. In 2008 the WHO implemented the MPOWER policy as a worldwide tobacco control strategy, aiming to assist in the country-level implementation of effective interventions to reduce the demand for tobacco.
and support as an integral aspect of routine patient care. Since its inception the OMSC has continued to expand. In 2020 the OMSC had been implemented in over 500 outpatient, inpatient and primary care sites across Canada. A 2015 study reported that 35% of patients who received the OMSC interventions were smoke-free at 6 months, and had a 40% reduction in risk of death over 2 years.11
during the daily safety-pause, and a poster campaign on the pilot ward. During the pilot programme, smoking documentation rate increased to 85.7%, and 95% of tobacco users were referred to local smoking cessation services, a significant improvement from the reported pre-intervention results.
smoking cessation services significantly improved practice. This project was supported by encouragement of nursing staff during the daily safety-pause, and a poster campaign on the pilot ward. During programme, smoking documentation rate increased to 85.7%, and 95% of tobacco users were referred smoking cessation services, a significant improvement from the reported pre-intervention results
In 2004, Ireland became the first country to pass a smoke-free law,8 and furthermore in 2021 was recognised by the WHO as a global leader in tobacco control. The Department of Health has set the goal of being tobacco-free by 2025, defined as a smoking prevalence of less than or equal to 5%. However, as the HI Survey shows, ongoing improvements in smoking cessation policy and practise are required to maintain progress towards this goal.
In Ireland, the HSE implemented the National Stop Smoking Guideline (NSSG) in 20227 aimed at improving the identification and treatment of tobacco addiction across all healthcare settings. Healthy Ireland reports that 29% of smokers are either trying to quit or are actively planning a stop date.4 However, only 18% of smokers who saw their GP over a 12-month period discussed methods of smoking cessation, a decline of 50% compared to data from 2019.4
HSE implemented the National Stop Smoking Guideline (NSSG) in 2022 (7) aimed at improving identification and treatment of tobacco addiction across all healthcare settings. Healthy Ireland reports smokers are either trying to quit or are actively planning a stop date (4) However, only 18% of saw their GP over a 12-month period discussed methods of smoking cessation, a decline of 50% data from 2019. (4)
As demonstrated in the HI Survey 2022, a large proportion of smokers suffer from chronic illness4 which in turn often results in hospital admissions and prolonged inpatient stays. This was demonstrated in a 2016 study which attributed 309,117 beddays to smoking related illnesses. The HSE recommends that each patient encounter in the hospital setting is used as a valuable opportunity for a brief intervention to discuss smoking cessation.
In 2017, the OMSC was adapted in the Greater Manchester Area in the UK as the CURE project; a comprehensive secondary care treatment program for tobacco addiction. The CURE project has been shown to be cost-effective, with a highly significant return on investment.12 The CURE project estimates a 50% reduction in readmissions at 30-days (3,273 admissions), and a reduction in re-admissions at 1 year from 38.4% to 26.7%. This project is estimated to save 30,880 bed days per year in the Greater Manchester Area. Furthermore, it has been proven to deliver high-quality care for patients by providing access to highly effective interventions and providing individualised smoking cessation treatment.13 The success of the CURE project has led to a commitment from the NHS that by 2023/24 all active smokers admitted to hospital will be offered NHS-funded tobacco treatment services, based on the successful and favourable results from the OMSC and CURE models.
recommends that all healthcare professionals ask and document an individual’s smoking behaviours, advise all active smokers about the harms of smoking and the benefits of cessation. Healthcare should discuss the individual treatment needs and preferences, and advise that making an quit attempt is less effective than using recommended supports. This is supported by WHO data demonstrates that brief advice from health professionals can increase quitting success by up to 30%, advice increases the chance of quitting by 84%.
In our service, this is particularly applicable in the Rapid Access Lung Clinic, a service for diagnosis and treatment of patients with suspected lung cancer. A recent analysis highlights the benefit to overall survival of stopping smoking at any time for a patient with lung cancer.9, 10
of health behaviour change that should be utilised by healthcare professionals in their day-to-day practice. Sometimes referred to as a ‘teachable moment’, these conversations aim to motivate individuals to adopt risk reducing health behaviours. The 5 A’s model (figure 2), recommended by the Centre for Disease Control (CDC) and WHO, summarises the activities that a healthcare provider can do within 3-5 minutes during a consultation. Multiple studies have shown the benefits of a brief intervention program, delivered by staff who are appropriately trained, with a statistically significant difference in smoking cessation and continued abstinence.14
The British Thoracic Society (BTS) and the National Institute for Health and Care Excellence (NICE) recommend that all frontline healthcare staff should receive training to identify smoking status and to offer very brief advice, as well as the local referral process for behavioural support.15,16
identifies asking about smoking behaviour and offering advice to quit as a health behaviour change that should be utilised by healthcare in their day-to -day practice. Sometimes referred to as a ‘teachable these conversations aim to motivate individuals to adopt risk reducing behaviours. The 5 A’s model (figure 2), recommended by the Centre for ontrol (CDC) and WHO, summarises the activities that a healthcare do within 3-5 minutes during a consultation. Multiple studies have benefits of a brief intervention program, delivered by staff who are trained, with a statistically significant difference in smoking continued abstinence. (14)
There is precedent for the implementation of smoking cessation frameworks within healthcare settings, as evidenced by the success of the Ottawa Model for Smoking Cessation (OMSC) which was first developed in the early 1990s. The OMSC is a validated, evidence-based process to embed comprehensive smoking cessation treatments
Thoracic Society (BTS) and the National Institute for Health and Care (NICE) recommend that all frontline healthcare staff should receive identify smoking status and to offer very brief advice, as well as the process for behavioural support. (15,16)
In Galway University Hospital we conducted a review of current smoking prevalence and smoking cessation services. An initial chart review revealed that 19.7% of audited patients were active smokers; overall in line with the national data. However, initially, only 64.4% of patients had smoking status documented, and only 13% received brief advice, with the same percentage referred to smoking cessation services. A standardised smoking cessation proforma was implemented on a pilot ward as part of the admission bundle, aiming to document smoking status on admission to hospital and encourage brief advice, and, in addition, all identified smokers were referred to smoking cessation services with their consent. These simple interventions including the introduction of a standardised approach to identification of smoking status and an optout referral of active smokers to smoking cessation services significantly improved practice. This project was supported by encouragement of nursing staff
The NSSG recommends that all healthcare professionals ask and document an individual’s smoking behaviours, and, in turn advise all active smokers about the harms of smoking and the benefits of cessation. Healthcare professionals should discuss the individual treatment needs and preferences, and advise that making an unsupported quit attempt is less effective than using recommended supports. This is supported by WHO data which demonstrates that brief advice from health professionals can increase quitting success by up to 30%, while intensive advice increases the chance of quitting by 84%.
The NSSG identifies asking about smoking behaviour and offering advice to quit as a key element

options for behavioural support include individual or group telephone or text-message support Currently, behavioural support specialist smoking cessation officers, both in hospital and based facilities, who can also provide prescription for NRT if Novel methods of delivering counselling for smoking cessation have been developed including the counselling service ‘Florence’, an artificial intelligence bot developed by the WHO. Florence is a 24/7

Additional options for behavioural support include individual or group counselling, telephone or text-message support. Currently, behavioural support is available specialist smoking cessation officers, both in hospital and community-based facilities, who can also provide prescription for NRT if required. Novel methods of delivering counselling for smoking cessation have been developed including the digital counselling service ‘Florence’, an artificial intelligence bot developed by the WHO. Florence is a 24/7 virtual health worker, capable of providing brief conversations by voice or text, and to signpost patients to other digital cessation programmes in their country, and can found at https://www.who.int/campaigns/ Florence. This technology was developed in 2021, primarily aimed at overcoming the barriers arising from the COVID-19 pandemic such as easy access to smoking cessation services. The HSE has also developed a digital cessation service, and offers a 28-day plan including personalised daily support via email and text message through the QUIT.ie website, with a personalised web page to track progress.
For patients who wish to use pharmacological therapy in combination with behavioural supports, the NSSG recommends that combination NRT treatment or NRT monotherapy should be prescribed. NRT is available in multiple forms and prescription can be tailored to the patient’s preference (figure 3). A recent Cochrane review confirmed the effectiveness of NRT in smoking cessation.17
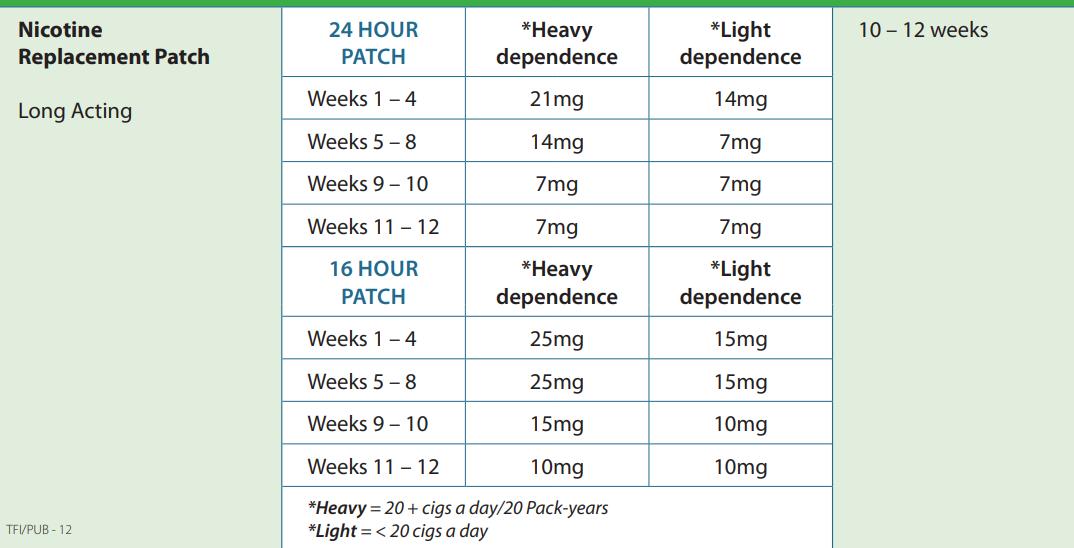
Figure 3: Stop Smoking medications recommended by the HSE National Clinical Guideline
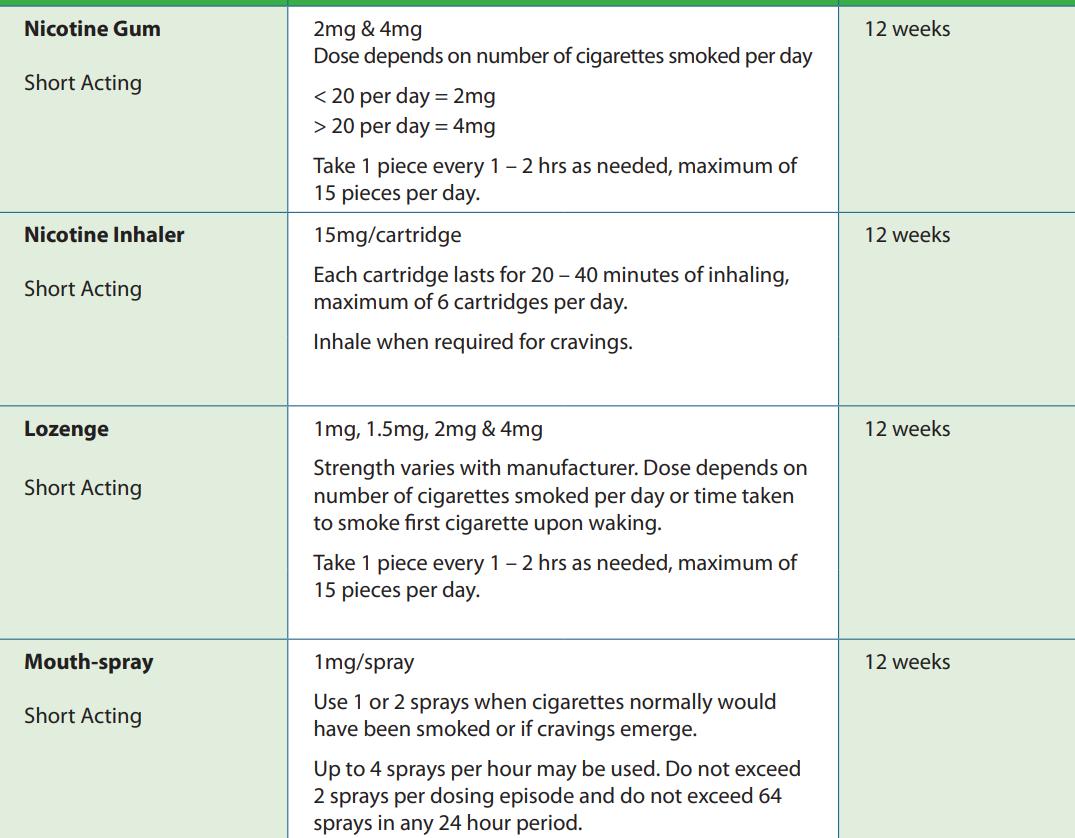
dependent on adequate access to behavioural and pharmacological supports for all patients, irrespective of chronic illness or socioeconomic status.
Conclusion
The development of an effective smoking cessation framework in the hospital setting has been proven by the Ottawa Model (Canada) and the CURE project (UK) to be a cost-effective intervention, which benefits patients at any stage of a disease course, and in addition can equal the benefit of costly pharmacological or surgical interventions. Recent data
indicates a slowdown in the momentum towards the target of a tobacco-free Ireland by 2025. Meeting this goal is dependent on accelerating progress with smoking cessation programs across all healthcare settings. Additional challenges are also appearing, for example, the smoking habits of Irish people are evolving with the increasing popularity of e-cigarettes and this will require the adaptation of current smoking cessation practise and
legislation to tackle these changes particularly amongst younger people. Providing standardised clinical care to patients in healthcare is challenging, due to the diversity in environments of care and patient presentations. However, there is an opportunity during all patient encounters to provide a brief intervention and in turn direct patients to appropriate smoking cessation treatments. Improvements in smoking cessation services in Ireland are
At present, we risk reversing the gains which have been made to date in Ireland towards the goal of a tobacco-free nation. The impact of the COVID-19 pandemic, novel nicotine delivery systems, and the rise of tobacco addiction among young people cannot be underestimated, and additional funding and training of healthcare professionals is required for smoking cessation services to maintain momentum in tackling tobacco addiction in Ireland.
References available on request
The Cardiothoracic team at University Hospital Galway have won a HSE Excellence Award for their project ‘Minimally Invasive Vessel Harvesting for Coronary Artery Bypass Grafts’ in the Innovation in Service Delivery Category on 28th November 2024.
The awards are an opportunity to showcase and celebrate examples of the great work that happens every day across our health service. They aim to encourage and inspire health service staff to develop and improve care and services for patients.
Describing the project Dr Sadiq Siddiqui, Associate Specialist in Cardiothoracic Surgery at University Hospital Galway said, “Part of our job is to perform coronary artery bypass surgery which requires harvesting veins and radial artery to perform this surgery.
“Traditionally those arteries and veins were harvested via a long incision which are associated with pain, wound infection, debilitating scars and delayed mobility
“For the last eight years we have been harvesting the radial artery and long saphenous vein via a 2cm incision and this technique has shown that the wound infection rate has reduced from 7% to 0%, while also maintaining excellent clinical outcomes and improving patient satisfaction from the cosmesis of
Cardiothoracic 1: From left, Sharon Umeh, Staff Nurse; Sharon Collins, Clinical Nurse Manager 1; Emer Lambe, Staff Nurse; Dr Sadiq Siddiqui, Associate Specialist in Cardiothoracic Surgery; Patient Bernadette Benagh; Annie Flaherty, Health Care Assistant and Niamh McDermott, Clinical Nurse Manager 2
their wound and the avoidance of post-operative complications.”
Speaking about her experience, Patient Bernadette Benagh said, “I am so honoured and privileged to be able to tell you about my experience. It was a wonderful, wonderful experience from the day I started until the day I finished and the way I am now is because of these people who saved my life.”
Welcoming the news, Hospital Manager Chris Kane said, “This technique is another enhancement in cardiac surgery resulting in better patient outcomes.
“We are enormously proud of the team and the recognition they have received by winning an HSE Excellence Award; it is well-deserved.”
Bernard Gloster, HSE CEO, commending the winners, said, “These awards create the opportunity to show innovation and creativity, which ultimately improves the services that we provide. Well done and thank you to all those short-listed as well as the winners. It is your work that helps pave the way to a brighter future for Ireland’s health service.”
Anne Marie Hoey, HSE Chief People Officer, highlighting the positive outcomes achieved, said, “As well as providing an opportunity to recognise the great work of staff and teams across our wide range of services, the Excellence Awards is an important channel to share learnings with our colleagues across the health service.
“Through this pooling of knowledge and expertise, the projects inspire a sense of staff pride, teamwork and collaboration. This will positively impact the overall environment for our staff whilst improving outcomes for the people who rely on our care and support.”

Children’s Health Ireland (CHI), in partnership with St. James’s Hospital have successfully opened Ireland’s first early phase clinical trial, enrolling eligible children with Duchenne muscular dystrophy (DMD). The global trial, called DELIVER, which started in August 2022 as the first-in-human (phase 1/2) trial of a therapeutic called DYNE-251 at sites around the world targets male children with a specific type of DMD (children amenable to exon 51 skipping). The trial is being delivered by the HRB CHI Clinical Research Centre and the Wellcome HRB Clinical Research Facility at St James’s Hospital and involves delivery of a next generation molecular therapy developed by Dyne Therapeutics, a Massachusetts based biotechnology company, specialising in the development of therapies for rare muscle diseases. The study is progressing through at sites around the world but is the first time such an early phase trial has been conducted in children in Ireland.
Duchene muscular dystrophy (DMD) is a rare neuromuscular disease that results in progressive deterioration in muscle strength and premature death. Symptoms are often first detected as early as age two, but diagnosis often occurs between four and five years of age. The disease is almost exclusively seen in boys. It is caused by gene mutations that affect a protein called dystrophin, which plays a critical role in muscle cell structure and function. As they grow, children with DMD become gradually weaker, losing the ability to walk and ultimately being confined to a wheelchair. Weakness of the muscles involved in swallowing, breathing and heart function are particularly serious and ultimately life threatening. Currently, very few treatments are available for this devastating disease, and children and their families are anxiously awaiting treatments that can target the basic defect in DMD, aimed at slowing or stopping the inevitable decline in muscle function.
Clinical trials can be extremely complex to undertake and are governed by very strict and detailed safety codes. They involve years of careful planning by a large team of experts within the hospitals, regulators and industry. Global healthcare regulatory agencies comprehensively review data on investigational medicines before
the clinical trial can start in humans.
Dr Declan O’Rourke, Consultant Paediatric Neurologist and principal investigator for the study at CHI, said: “This is a watershed moment for CHI. We have been working for many years to increase the number of trials we offer to our patients with progressive muscle diseases and have had some great successes already. DMD is a condition with very few treatment options currently, and with this trial, it is great to be at the very forefront of potentially bringing new, transformative therapies to children.”
The development of clinical trials in Neurology in CHI has been supported by a research grant from Children’s Health Foundation, CHI’s fundraising partner.
CHI’s Director of Research & Innovation, Paul McNally welcomed CHI’s participation in the ongoing study saying:
“This is an exciting day for everyone involved in clinical trials in CHI. These early phase trials are an enormous undertaking but a vitally important thing for us to be able to do if we really want to become a leading children’s hospital and offer our patients the very latest in cutting edge treatments in the world. Our partnership with St James’s Hospital has been critical here and is an example of the type of benefits that we can expect when working together on the same campus when we move to the new children’s Hospital.”
DON’T LET STIGMA BE A BARRIER TO SEEKING HELP FOR MENTAL HEALTH DIFFICULTIES
Some 20% of adults in Ireland would tell no one if they were experiencing suicidal thoughts, St Patrick’s Mental Health Services’ 2024 annual Attitudes to Mental Health and Stigma Survey has revealed. The survey also found a significant 49% of people who have experienced mental health difficulties have not sought treatment due to stigma or embarrassment. In light of these findings, St Patrick’s Mental Health Services is urging everyone to check in on their own and their loved ones’ mental health and wellbeing, particularly during the Christmas period when the pressures of the season can often take over, and to seek support if needed.
For over 10 years, St Patrick’s Mental Health Services has
commissioned this annual survey to track changing attitudes towards mental health and stigma. While there have been improvements in attitudes, the 2024 findings highlighted that stigma is still a significant barrier to seeking help, with shame or embarrassment often impacting willingness to disclose mental health difficulties and to engage in treatment. For example, this year’s survey, which polled a nationally representative of over 1,000 adults in the Republic of Ireland, found:
- 53% of respondents have experienced a mental health difficulty, yet more than one-third (34%) did not seek treatment, with the main reason for not doing so cited as shame.
- 23% of people would consider it a sign of weakness if they sought help for a mental health difficulty. At the same time, 11% would consider it a sign of weakness if a friend or loved one sought help, indicating the prevalence of self-stigma.
Speaking about the findings, Paul Gilligan, CEO of St Patrick’s Mental Health Services said:
“While attitudes towards mental health are improving in many ways, as reflected year-on-year in our annual survey findings, one concerning area that has seen little change is in the disclosure of suicidal thoughts and the idea that it’s a sign of weakness to seek help for your mental health.
Speaking up about your mental health takes courage. Oftentimes, a person might feel like a burden to others or that mental health difficulties may reflect a personal weakness. This is absolutely not the case, and we need to continue to challenge these deeply ingrained attitudes; to continually demonstrate the strength in speaking out and seeking help and reinforce the possibility of recovery from mental health difficulties with the right support.
In the busyness of the holiday season, it’s important to remember that the most important thing we can do with our time is to connect with our emotional selves and to check in on how we, and others, are really feeling. If we ourselves, or someone we love, is struggling, don’t let stigma stand in the way of seeking support.”
Despite some stagnated attitudes towards stigma, findings from the 2024 survey have also shown areas where attitudes to mental health are notably improving, for example:
• Less people are afraid of experiencing mental health difficulties themselves in the future than they were five years ago, indicating increased understanding of mental health difficulties and awareness that recovery from mental health difficulties is possible.
• Since 2020, there has been a 16% decrease in the number of people who believe that being treated for a mental health difficulty is still seen by Irish society as a sign of personal failure. In 2020, 64% of respondents agreed with this statement while this figure stood at 48% in 2024.
• 66% of people who experienced mental health difficulties in 2024 sought treatment – a 10% increase since 2023.
• 78% of this year’s survey respondents also believe that people with mental health difficulties experience less stigma and discrimination than 10 years ago.
• 52% have had a positive experience of disclosing mental health difficulties at work, at home or in the local community.
MSD IRELAND WELCOMES LAUNCH OF “ACTION PLAN TO ELIMINATE HPV-RELATED CERVICAL CANCER” IN IRELAND BY 2040
MSD Ireland welcomes the news that cervical cancer rates in Ireland continue to drop as Ireland launches an ambitious Cervical Cancer Elimination Action Plan, a “significant milestone in the global drive to eliminate cervical cancer by 2040.” The newly launched action plan aligns with MSD’s own commitment to harnessing the power of leading-edge science to address some of the world’s biggest healthcare challenges, including the elimination of HPV related cervical cancer.
Cervical cancer remains one of the most common cancers affecting women globally, with an estimated 265 cases diagnosed annually in Ireland alone, and 85 lives lost each year to this disease. Yet, with a combination of increased vaccination, enhanced screening, and more comprehensive treatment, we can work towards a future where no woman must go through the distress of a cervical cancer diagnosis in Ireland.
This Action Plan is launched on the fourth anniversary of the World Health Organisation (WHO) global initiative to eliminate
cervical cancer and one year on from the Minister for Health's announcement of Ireland's commitment to this goal, setting the target date for cervical cancer elimination by 2040. Ireland joins countries like Sweden and Australia, which are already on track to eliminate the disease.
Samantha Humphreys, Managing Director of Human Health at MSD Ireland, commented: “We welcome the introduction of the Cervical Cancer Elimination Action Plan in Ireland as a significant milestone in the global drive to eliminate cervical cancer. Ireland has an important role to play and we’re immensely proud of the role we play in supporting the goal of HPV elimination, starting with cervical cancer elimination.”
She continued: “The introduction of this action plan marks a crucial step forward in reducing the burden of the disease and improving long-term health outcomes for women. The action plan represents a vital step in Ireland's effort to eliminate the impact of the disease, through a focus on screening and vaccination programmes, and ultimately saving lives by preventing future cases of cervical cancer. To achieve this ambitious goal, stakeholders must work together on this action plan, and the specific goals outlined within, which includes: ‘increasing HPV vaccination rates for girls by age 15 to 90% by 2030 (currently at 84%); maintaining cervical screening coverage above 70% (currently at 73%); and maintaining the number of women receiving treatment above 90% (currently at 97%). We particularly welcome and will support efforts to increase awareness and education about HPV and related cancers in the community and among vulnerable and marginalised groups.”
In anticipation of the full implementation of the six new health regions in early 2025, the NCRI annual report on national cancer statistics has, for the first time, examined cancer incidence and survival3-6 across these six geographies for the four most common cancers in Ireland: lung, bowel (colorectal), breast and prostate cancer. These data are presented alongside national statistics providing an annual update on the status of cancer in Ireland.
In Ireland as a whole, an average of 44,000 tumours were diagnosed each year during 2020-2022. Prof.
Deirdre Murray, Director of NCRI, and Professor of Epidemiology at University College Cork, noted that the majority of these (24,200) were potentially life-changing invasive cancers requiring extensive treatment. On average 9,800 people died each year from cancer during 2020-2022. One in five deaths were due to lung cancer.
Considering the six new health regions, the majority (60%) of all invasive cancers diagnosed during 2020-2022 occurred in the three eastern geographical areas (Dublin and North East, Dublin and Midlands, and Dublin and South East). Compared to the national average, the lung cancer incidence rate was higher in Dublin and Midlands, and lower in Dublin and South East. By contrast, the rate of prostate cancer was higher in Dublin and South East, but lower in Dublin and North East.
The proportion of people diagnosed during 2009-2018 and surviving at 5-years following diagnosis was lower than the national average for those living in the Mid West for colorectal, lung, and female breast cancer. Fiveyear survival for prostate cancer was also lower than the national average for people resident in the Dublin and North East region at the time of their diagnosis.
The report also found that, in Ireland as a whole, the number of people living with and beyond cancer continues to grow with an estimated 220,700 people who had been diagnosed with cancer still alive at the end of 2022 (about 1 in 23 of the Irish population).
Prof. Murray, said: “One of the core functions of the NCRI is to provide data for cancer service planning, evaluation and policymaking. While geographic variation must always be interpreted with care these data can point to important opportunities for improvement across cancer care from prevention, early diagnosis, and screening, to access to services and treatment.”
Chair of the NCRI Board, Dr Robert O’Connor added: “This report provides a comprehensive overview of cancer statistics as our country began to recover from the COVID-19 pandemic. The data reveal critical insights into regional health outcome differences and emerging trends in cancer survival rates. The findings underscore the continued need for targeted, strategic investments in cancer research, prevention, treatment, and post-treatment care to equitably maximise patient outcomes and community health.”
THE HEALTH SERVICE EXECUTIVE (HSE) APPROVES FINTEPLA®(FENFLURAMINE) FOR PATIENTS IN IRELAND WITH DRAVET SYNDROME AND LENNOX GASTAUT SYNDROME (LGS)
UCB is pleased to announce that the Health Service Executive (HSE) has approved FINTEPLA® (fenfluramine) for the treatment of Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS) in patients from two years of age. This reimbursement has allowed eligible patients to access fenfluramine much sooner than expected under the routine approval processes.
Nadeem Aurangzeb, Head of Rare & Epilepsy, UCB UK & Ireland, said: “We are immensely pleased that fenfluramine has been approved by the HSE. We know that Irish patients and their families have been looking forward to accessing this treatment, due to the burden that Dravet syndrome and Lennox Gastaut syndrome places on the lives of those living with these complex, rare conditions. We’re also pleased that Irish patients will be able to access fenfluramine over a year ahead of the expected approval date. This approval means fenfluramine is available to Irish patients ahead of those in the rest of the UK, which is almost unheard of.”
LGS is a rare, severe, earlyonset developmental epileptic encephalopathy, which is characterised by multiple types of seizures, intellectual impairment and electroencephalography (EEG) abnormalities. It affects around 0.1 to 0.28 people per 100,000 and it accounts for 1-10% of childhood epilepsies and 1-2% of all epilepsies.
The approval of fenfluramine for the treatment of LGS-associated seizures is based on phase 3 data and a long-term open-label extension study.4,5 In the phase 3 trial, 263 patients ages two to 35 years were randomised to receive either fenfluramine or placebo.
Patients assigned to fenfluramine experienced a significantly greater reduction in drop seizures, compared with the placebo group. The most frequent adverse events included decreased appetite, somnolence, and fatigue, and there were no cases of valvular heart disease (VHD) or pulmonary arterial hypertension (PAH). Furthermore, in the open-label extension study, patients taking fenfluramine continued to
experience sustained (39.4% 51.8%, 28.6% overall) reductions in drop seizure frequency. The treatment was generally well-tolerated and long-term VHD and PAH was not observed.5 DS, another developmental epileptic encephalopathy, is also a rare and life-long form of epilepsy that begins in infancy and is marked by frequent seizures.
People living with DS experience significant developmental and motor impairments that persist into adulthood and quality of life is severely impacted for patients, their families, and caregivers due to its high physical, emotional, and financial burden.
Data from a randomised, double-blind, placebo-controlled phase 3 trial supports the use of fenfluramine in DS, where 119 patients received either fenfluramine or placebo.10 Fenfluramine provided significantly greater reduction in convulsive seizure frequency, compared with the placebo group. It was also generally well-tolerated, with no observed cases of VHD or PAH.
People living with both LGS and DS have an increased risk of sudden death, known as Sudden Unexpected Death (SUDEP), and it is estimated that there are over 130 epilepsy-related deaths in Ireland per year.
Nicola Kehoe of Dravet Syndrome Ireland said: “We are delighted to have fenfluramine available as a funded treatment for people living with Dravet syndrome and Lennox Gastaut Syndrome in Ireland. We know from the international community that fenfluramine is very effective in reducing seizures and we welcome the potential improvements in health and quality of life for our community of families living with these very complex epilepsies.”
Fenfluramine is currently only available to people living with LGS in Ireland, however, UCB is working collaboratively to secure reimbursement for patients living in England, Wales and Scotland. Marketing authorisation was previously obtained from the European Medicines Agency (EMA) in 2020, and approval was obtained from the European Commission (EC) in 2023.
The Medicines and Healthcare products Regulatory Agency (MHRA) authorised fenfluramine for the treatment of seizures associated with DS and LGS in July 2023.

Pictured at the Novartis and Deciphex AI event are (from left): Caitriona Walsh, Country President, Novartis Ireland, Dr. Donal O’Shea, CEO of Deciphex and Laura Kavanagh, Research & Advocacy Manager, the Irish Platform for Patients’ Organisations (IPPOSI). Pictured taking part in the panel discussion are (from left); Moderator Elaine Burke; Minister of State for Digital Dara Calleary TD; Laura Kavanagh and Neasa McNabola, Senior Scientific Adviser, IDA BioPharma Team
NOVARTIS AND DECIPHEX HOST ROUNDTABLE ABOUT IRELAND’S AI SECTOR TO MARK COLLABORATION
Novartis Ireland has marked its Collaboration Agreement with Deciphex, a Dublin-based digital pathology company, at a specially convened roundtable on Artificial Intelligence (AI).
The collaboration is a global agreement between Novartis and Deciphex. Harnessing cuttingedge technology, it seeks to develop a suite of AI tools that will improve the efficiency and accuracy of preclinical studies and accelerate the painstaking work of drug discovery and development. Ultimately, this collaboration has the potential to positively impact patient pathways.
In Ireland, Novartis and Deciphex marked the agreement at a panel discussion titled ‘How Ireland can be the Centre of Artificial Intelligence (AI) for Europe’. Taking place at Novartis Ireland and officiated by Minister of State for Digital Dara Calleary TD, the event featured contributions from Novartis and Deciphex leadership and representatives from IDA Ireland and patient-led platform, IPPOSI.
The event heard from panelists that government policies and
State support, ongoing investment and a strong pipeline of tech talent from universities are key factors in creating a fertile ecosystem where the AI sector in Ireland is flourishing.
Against this backdrop, partnerships between indigenous firms and multinationals such as the Novartis and Deciphex collaboration, can deliver positive economic, clinical and societal outcomes in the coming years.
The roundtable also heard that with AI set to transform sectors such as healthcare over the coming years, the public and patients are being engaged to ascertain the degree in which safeguards and regulation can provide comfort that with AI advances, a human will still be in the loop. The Irish public are being invited to share their insights for policymakers through important initiatives such as the IPPOSI ‘Citizens’ Jury on AI in Healthcare’.
Minister of State for Digital Dara Calleary TD said: “I am delighted to officiate at today’s panel discussion. Ireland’s AI sector is flourishing, with a partnership approach between State bodies, enterprise and the public key to delivering transformative societal benefits in the coming years. The Novartis-Deciphex collaboration is a great example of how this approach works in practice, with an indigenous medtech collaborating with a global leader in pharma to harness cutting-edge technology and deliver better outcomes for patients.”
Michael Lohan, CEO, IDA Ireland said, “Artificial Intelligence is a cornerstone of Ireland’s strategy to drive innovation and fuel economic growth. By fostering a robust AI ecosystem, we are not only attracting leading global tech companies but also empowering local enterprises to compete on the world stage. The integration of
AI across various sectors is pivotal in enhancing productivity, creating high-value jobs, and ensuring Ireland remains at the forefront of technological advancements”
Dr. Donal O’Shea, CEO of Deciphex said: “We are thrilled to collaborate with Novartis in leveraging AI technology to drive innovation in preclinical research. This partnership underscores our commitment to advancing healthcare through the development of cutting-edge solutions that address critical challenges in drug development lifecycles. Novartis is committed to innovating AI-based approaches with potential to accelerate drug discovery and development and bring life-changing medicines to patients faster.” Statement approved by stakeholder
Caitriona Walsh, Country President, Novartis Ireland said: “I’m excited that Novartis has entered into a collaboration agreement with Deciphex to develop Artificial Intelligencebased approaches aimed at improving the efficiency and accuracy of preclinical studies. We believe AI has transformative potential to accelerate the painstaking work of drug discovery and development. I look forward to seeing how our collaborative efforts can help us improve and streamline existing processes to deliver impactful medicines to patients more quickly.”
HSE APPROVES FINTEPLA®
UCB is pleased to announce that the Health Service Executive (HSE) has approved FINTEPLA® (fenfluramine) for the treatment of Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS) in patients from two years of age. This reimbursement has allowed eligible patients to access fenfluramine much sooner than expected under the routine approval processes.
Nadeem Aurangzeb, Head of Rare & Epilepsy, UCB UK & Ireland, said: “We are immensely pleased that fenfluramine has been approved by the HSE. We know that Irish patients and their families have been looking forward to accessing this treatment, due to the burden that Dravet syndrome and Lennox Gastaut syndrome places on the lives of those living with these complex, rare conditions. We’re also pleased that Irish patients will be able to access fenfluramine over a year ahead of the expected approval date. This approval means fenfluramine is available to Irish patients ahead of
those in the rest of the UK, which is almost unheard of.”
LGS is a rare, severe, earlyonset developmental epileptic encephalopathy, which is characterised by multiple types of seizures, intellectual impairment and electroencephalography (EEG) abnormalities. It affects around 0.1 to 0.28 people per 100,000 and it accounts for 1-10% of childhood epilepsies and 1-2% of all epilepsies.
The approval of fenfluramine for the treatment of LGS-associated seizures is based on phase 3 data and a long-term open-label extension study. In the phase 3 trial, 263 patients ages two to 35 years were randomised to receive either fenfluramine or placebo. Patients assigned to fenfluramine experienced a significantly greater reduction in drop seizures, compared with the placebo group. The most frequent adverse events included decreased appetite, somnolence, and fatigue, and there were no cases of valvular heart disease (VHD) or pulmonary arterial hypertension (PAH).
Furthermore, in the open-label extension study, patients taking fenfluramine continued to experience sustained (39.4% 51.8%, 28.6% overall) reductions in drop seizure frequency. The treatment was generally welltolerated and long-term VHD and PAH was not observed.5 DS, another developmental epileptic encephalopathy, is also a rare and life-long form of epilepsy that begins in infancy and is marked by frequent seizures.
People living with DS experience significant developmental and motor impairments that persist into adulthood and quality of life is severely impacted for patients, their families, and caregivers due to its high physical, emotional, and financial burden.
Data from a randomised, double-blind, placebo-controlled phase 3 trial supports the use of fenfluramine in DS, where 119 patients received either fenfluramine or placebo. Fenfluramine provided significantly greater reduction in convulsive seizure frequency, compared with the placebo group. It was also generally well-tolerated, with no observed cases of VHD or PAH.
People living with both LGS and DS have an increased risk of sudden death, known as Sudden Unexpected Death (SUDEP), and it is estimated that there are over 130 epilepsy-related deaths in Ireland per year.
Nicola Kehoe of Dravet Syndrome Ireland said: “We are delighted to have fenfluramine available as a funded treatment for people living with Dravet syndrome and Lennox Gastaut Syndrome in Ireland. We know from the international community that fenfluramine is very effective in reducing seizures and we welcome the potential improvements in health and quality of life for our community of families living with these very complex epilepsies.”
Fenfluramine is currently only available to people living with LGS in Ireland, however, UCB is working collaboratively to secure reimbursement for patients living in England, Wales and Scotland. Marketing authorisation was previously obtained from the European Medicines Agency (EMA) in 2020, and approval was obtained from the European Commission (EC) in 2023. The Medicines and Healthcare products Regulatory Agency (MHRA) authorised fenfluramine for the treatment of seizures associated with DS and LGS in July 2023.
Sligo University Hospital is delighted to announce the opening of the Medical Offsite Ward, a new 26-bed ward comprising of six four-bed rooms and two single ensuite rooms, located at St John’s Community Hospital, Sligo.
This new development is an extension of Sligo University
The new 26-bed Medical Offsite Ward which is an extension of Sligo University Hospital will provide additional bed capacity and improved accommodation for patients
Hospital and will provide additional bed capacity, improved accommodation for patients and will ease patient flow from the Emergency Department and throughout the hospital, while meeting the required national standards and delivering enhanced quality care to patients.
The additional beds were part of the capital funding from the HSE 2024 Capital Plan, costing in excess of ¤2.5m for the design, build and equipping of the ward.
The medical offsite ward which is a specialised facility will provide structured medical support to patients who meet the criteria for the ward and will continue to benefit from medical supervision and therapeutic support, provided by a full team of health and social care professionals in a calm environment. It is suitable for patients who would benefit from an additional few days under the care of hospital staff.
Grainne McCann, Hospital Manager, stated, "This new development is a welcome addition to the hospital, allowing us to expand our inpatient care in a new contemporary ward designed to provide personalised, compassionate care to support our patients' medical needs and recovery during their inpatient stay."
Patient, Elizabeth Fallon, said, “When I came to the new medical ward, I was flabbergasted with the space and facilities. Everybody here is fantastic and the atmosphere is great. Everyone was very welcoming and cheerful on my arrival to the ward. The physios are fantastic. Last night was the first night I got a good sleep. My son who came into see me was also amazed with the new environment.”